Final ID: FR497
Efficacy and Safety of Lorundrostat in Hypertension Patients with High Unmet Medical Need: Subgroup Analyses of the Launch-HTN Trial in Uncontrolled and Treatment-Resistant Hypertension
Abstract Body: Background
The burden of uncontrolled hypertension and related cardiorenal morbidity and mortality is disproportionately higher in Black/African Americans (AA), adults aged ≥65 years, women, and those with comorbid obesity (BMI ≥30). In these cohorts, aldosterone dysregulation is an important contributor to their hypertension disease process. In addition, obesity independently contributes to aldosterone dysregulation, making inhibition of aldosterone biosynthesis an attractive treatment target in these patients. The Launch-HTN trial evaluated blood pressure (BP) lowering efficacy and safety of the highly selective aldosterone synthase inhibitor lorundrostat in a diverse participant population, including these subgroups with a high risk of future cardiovascular and kidney disease.
Objective
To assess BP lowering efficacy and safety of lorundrostat in Black/AA, age ≥65 years, women and obese subgroups in the Launch-HTN trial.
Methods
Launch-HTN was a global, phase 3 trial in adults with uncontrolled HTN, including treatment-resistant hypertension (rHTN). Participants taking 2 to 5 prescribed antihypertensive medications including a diuretic and with automated office systolic BP (AOSBP) of 135-180 mmHg and diastolic BP of 65-110 mmHg were randomized to receive once daily placebo (n=270) or lorundrostat 50 mg (n=808). The primary endpoint was change in AOSBP compared with placebo after 6 weeks of treatment. We assessed the BP lowering efficacy of lorundrostat among these pre-specified participant subgroups. Safety was assessed as the incidence and severity of adverse events (AEs).
Results
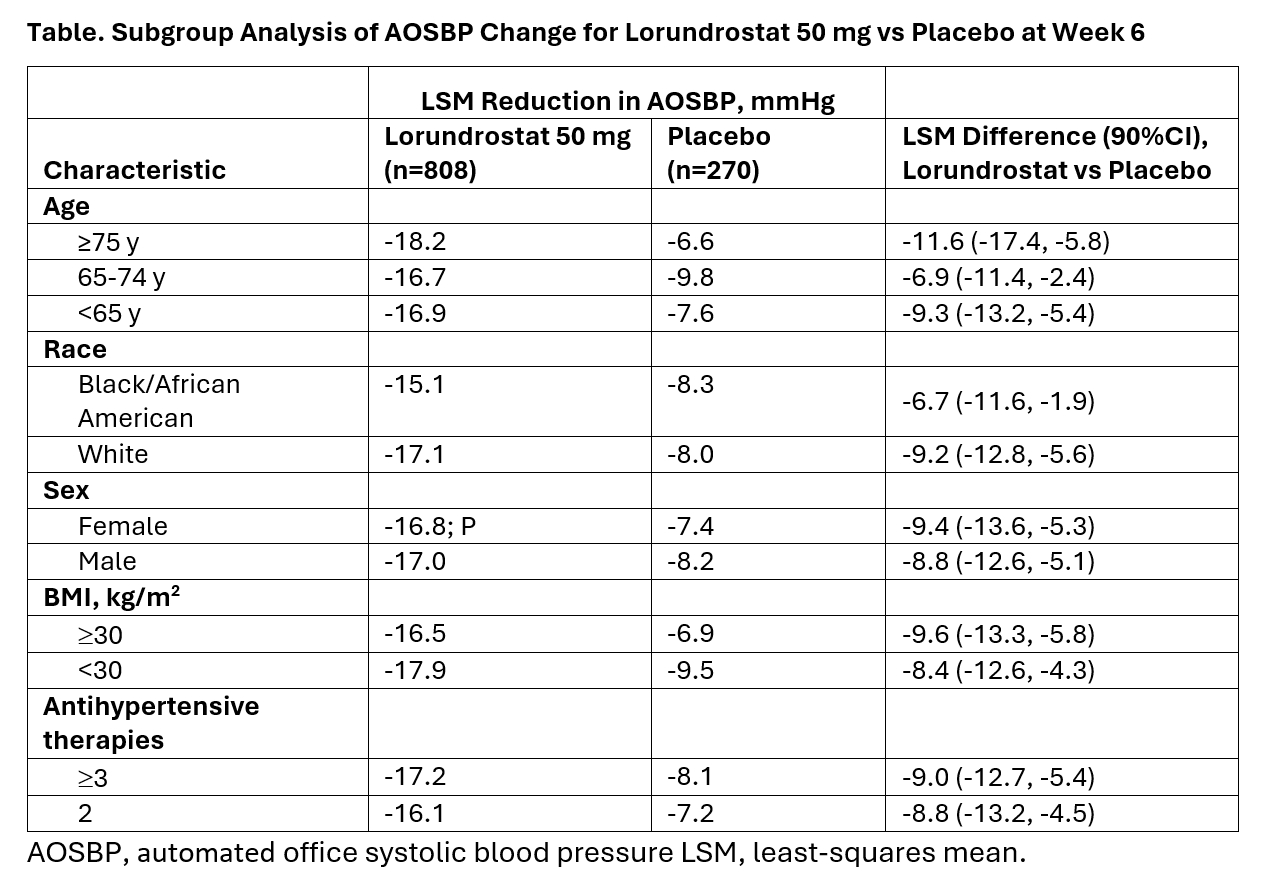
Launch-HTN included 29% Black/AA, 41% ≥65 years old, 47% were women and 63% obese participants. Across these subgroups, least-square mean differences (90% CI) in AOSBP reduction at 6 weeks uniformly favored lorundrostat vs placebo (Table). LSM AOSBP reduction at Week 6 with lorundrostat 50 mg once daily ranged from 15.1 mmHg in Black/AA participants to 18.2 mmHg in adults aged ≥75 years.
Conclusions
Lorundrostat leads to significant AOSBP reduction across high-risk participant subgroups with uncontrolled HTN, including rHTN. Reducing aldosterone biosynthesis via aldosterone synthase inhibition is a viable treatment strategy across multiple subgroups of uncontrolled hypertension with high cardiovascular and kidney disease risk. If sustained with long-term treatment, this BP reduction would be expected to confer cardiovascular-renal protection in these high-risk patients.
The burden of uncontrolled hypertension and related cardiorenal morbidity and mortality is disproportionately higher in Black/African Americans (AA), adults aged ≥65 years, women, and those with comorbid obesity (BMI ≥30). In these cohorts, aldosterone dysregulation is an important contributor to their hypertension disease process. In addition, obesity independently contributes to aldosterone dysregulation, making inhibition of aldosterone biosynthesis an attractive treatment target in these patients. The Launch-HTN trial evaluated blood pressure (BP) lowering efficacy and safety of the highly selective aldosterone synthase inhibitor lorundrostat in a diverse participant population, including these subgroups with a high risk of future cardiovascular and kidney disease.
Objective
To assess BP lowering efficacy and safety of lorundrostat in Black/AA, age ≥65 years, women and obese subgroups in the Launch-HTN trial.
Methods
Launch-HTN was a global, phase 3 trial in adults with uncontrolled HTN, including treatment-resistant hypertension (rHTN). Participants taking 2 to 5 prescribed antihypertensive medications including a diuretic and with automated office systolic BP (AOSBP) of 135-180 mmHg and diastolic BP of 65-110 mmHg were randomized to receive once daily placebo (n=270) or lorundrostat 50 mg (n=808). The primary endpoint was change in AOSBP compared with placebo after 6 weeks of treatment. We assessed the BP lowering efficacy of lorundrostat among these pre-specified participant subgroups. Safety was assessed as the incidence and severity of adverse events (AEs).
Results
Launch-HTN included 29% Black/AA, 41% ≥65 years old, 47% were women and 63% obese participants. Across these subgroups, least-square mean differences (90% CI) in AOSBP reduction at 6 weeks uniformly favored lorundrostat vs placebo (Table). LSM AOSBP reduction at Week 6 with lorundrostat 50 mg once daily ranged from 15.1 mmHg in Black/AA participants to 18.2 mmHg in adults aged ≥75 years.
Conclusions
Lorundrostat leads to significant AOSBP reduction across high-risk participant subgroups with uncontrolled HTN, including rHTN. Reducing aldosterone biosynthesis via aldosterone synthase inhibition is a viable treatment strategy across multiple subgroups of uncontrolled hypertension with high cardiovascular and kidney disease risk. If sustained with long-term treatment, this BP reduction would be expected to confer cardiovascular-renal protection in these high-risk patients.
More abstracts on this topic:
Accuracy of Angiographic Identification of Culprit Lesions in Women with MINOCA
Shwayder Elianna, Li Vincent, Smilowitz Nathaniel, Serrano Claudia, Elbaum Lindsay, Shah Binita, Hochman Judith, Hausvater Anais, Reynolds Harmony
A Subtle Case of Primary AldosteronismAgarwal Nikita, Sinha Partha, Tung Patricia, Krawisz Anna