Final ID: MP364
Advance-HTN Trial Participant Characteristics Associated with a Failure to be Randomized
Abstract Body (Do not enter title and authors here): Background
Placebo or sham-controlled clinical trials of new hypertension therapies often use run-in periods to assess participant adherence to background antihypertensive (AHT) therapy, assess participant response to a standardized AHT regimen and/or measure out of office blood pressure (BP) prior to randomization to ensure true uncontrolled hypertension. When these factors are assessed a large proportion of participants are excluded prior to randomization which can negatively impact a trial’s ability to enroll in an efficient manner. The Advance-HTN trial demonstrated the BP lowering efficacy of lorundrostat compared with placebo. Participants taking 2 to 5 AHT medications were enrolled and then switched to a standardized AHT regimen for a 3-week run-in period. The standardized regimen included olmesartan, a diuretic (hydrochlorothiazide or indapamide) and in certain cases amlodipine (if participant was taking ≥3 medications at enrollment). Participants were subsequently randomized if BP was uncontrolled when 24-hour ambulatory BP was measured while taking the standardized regimen.
Objective
This post hoc analysis aims to understand which Advance-HTN participant characteristics were associated with a failure to be randomized among participants that were enrolled and participated in the standardized AHT regimen run-in period.
Methods
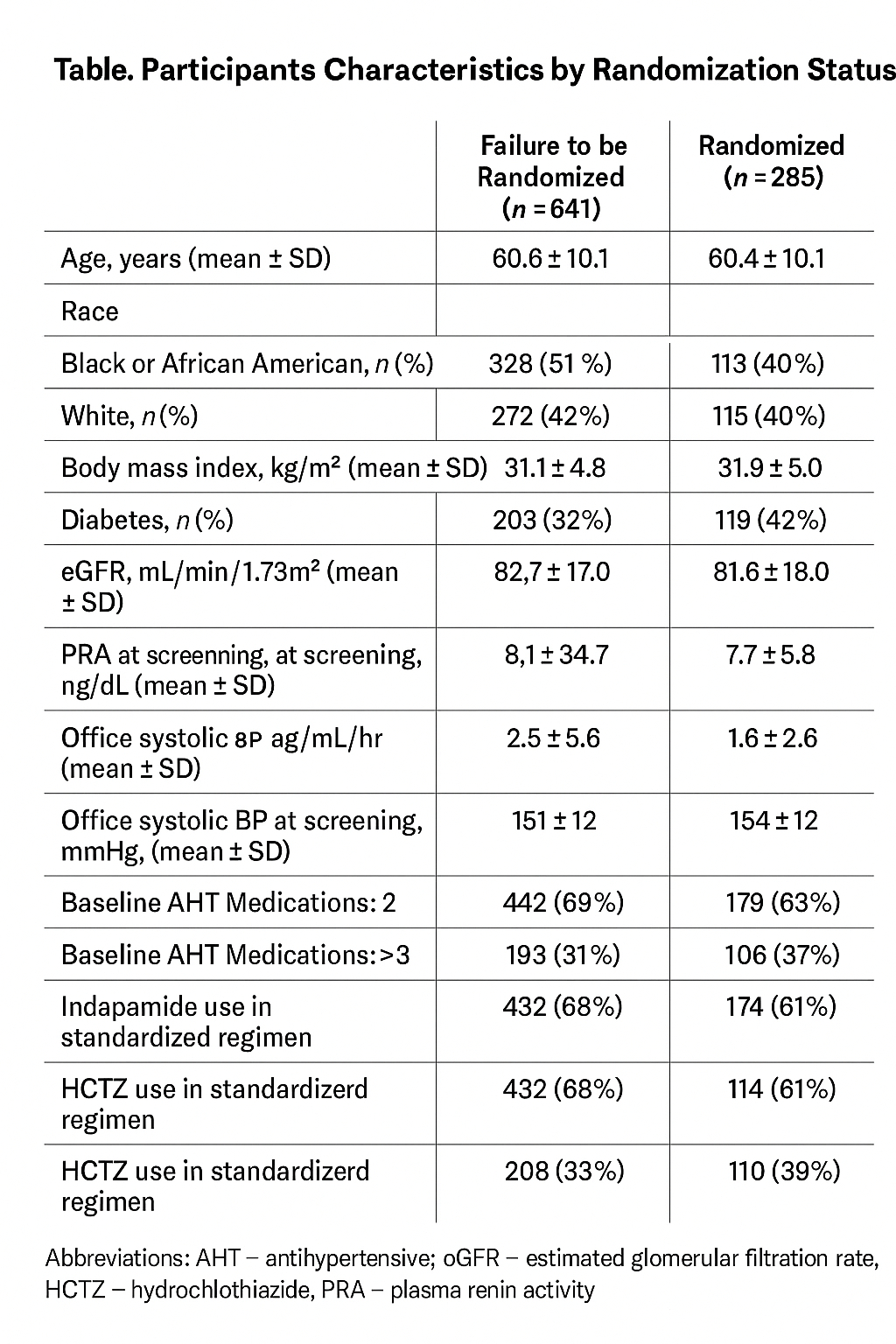
Multivariable logistic regression was performed comparing participants who took part in the run-in period and failed to be randomized with those participants that were successfully randomized. Candidate predictors included participant demographics, labs and medication use (Table).
Results
Of the 926 participants who started the standardized AHT regimen 285 proceeded to randomization. In the multivariable model, diabetes was associated with lower odds of failure to be randomized (OR: 0.61, 95% CI: 0.45-0.81, p < 0.001). Similarly, higher office systolic BP at screening was associated with lower odds of failure to be randomized (OR: 0.83 per 10 mmHg increase, 95% CI: 0.74-0.93, p < 0.001). In contrast, use of indapamide in the standardized AHT regimen was associated with greater odds of failing to be randomized (OR: 1.39, 95% CI: 1.03-1.87, p = 0.03).
Conclusion
Advance-HTN participant characteristics associated with a failure to be randomized were identified. These data may inform future hypertension trial design including participant selection strategies to maximize enrollment of patients with true uncontrolled hypertension
Placebo or sham-controlled clinical trials of new hypertension therapies often use run-in periods to assess participant adherence to background antihypertensive (AHT) therapy, assess participant response to a standardized AHT regimen and/or measure out of office blood pressure (BP) prior to randomization to ensure true uncontrolled hypertension. When these factors are assessed a large proportion of participants are excluded prior to randomization which can negatively impact a trial’s ability to enroll in an efficient manner. The Advance-HTN trial demonstrated the BP lowering efficacy of lorundrostat compared with placebo. Participants taking 2 to 5 AHT medications were enrolled and then switched to a standardized AHT regimen for a 3-week run-in period. The standardized regimen included olmesartan, a diuretic (hydrochlorothiazide or indapamide) and in certain cases amlodipine (if participant was taking ≥3 medications at enrollment). Participants were subsequently randomized if BP was uncontrolled when 24-hour ambulatory BP was measured while taking the standardized regimen.
Objective
This post hoc analysis aims to understand which Advance-HTN participant characteristics were associated with a failure to be randomized among participants that were enrolled and participated in the standardized AHT regimen run-in period.
Methods
Multivariable logistic regression was performed comparing participants who took part in the run-in period and failed to be randomized with those participants that were successfully randomized. Candidate predictors included participant demographics, labs and medication use (Table).
Results
Of the 926 participants who started the standardized AHT regimen 285 proceeded to randomization. In the multivariable model, diabetes was associated with lower odds of failure to be randomized (OR: 0.61, 95% CI: 0.45-0.81, p < 0.001). Similarly, higher office systolic BP at screening was associated with lower odds of failure to be randomized (OR: 0.83 per 10 mmHg increase, 95% CI: 0.74-0.93, p < 0.001). In contrast, use of indapamide in the standardized AHT regimen was associated with greater odds of failing to be randomized (OR: 1.39, 95% CI: 1.03-1.87, p = 0.03).
Conclusion
Advance-HTN participant characteristics associated with a failure to be randomized were identified. These data may inform future hypertension trial design including participant selection strategies to maximize enrollment of patients with true uncontrolled hypertension
More abstracts on this topic:
A Randomized Comparison of Online Motivational Themes in Cardiovascular Clinical Trial Recruitment
Hussain Zaib, Harry Tamunotonye, Michos Erin, Milller Hailey, Juraschek Stephen, Turkson-ocran Ruth-alma, Lahey Timothy, Feng Yuanyuan, Plante Timothy
Apabetalone Protects Against Heart Failure with Preserved Ejection Fraction by Suppressing Myocardial InflammationCostantino Sarah, Nazha Hamdani, Paneni Francesco, Gorica Era, Mohammed Shafeeq, Telesca Marialucia, Mongelli Alessia, Masciovecchio Valeria, Herwig Melissa, Ambrosini Samuele, Ruschitzka Frank