Final ID: Tu101
Measuring Fibrosis Progression in Duchenne Cardiomyopathy Using Cardiac Magnetic Resonance in mice
Abstract Body: Introduction: Transthoracic echocardiogram (TTE) is currently the gold standard for measuring cardiac function in mouse models of cardiomyopathy, however, it has its limitations. Challenges include poor acoustic windows, lower reproducibility, larger margin of error, and inability to accurately detect fibrosis. The detection of fibrosis is critical for assessing the severity of cardiac involvement and monitoring disease progression in Duchenne muscular dystrophy (DMD). Cardiac magnetic resonance imaging (CMR) can be used to more accurately measure cardiac function, chamber volumes, and tissue characterization to monitor the progression of fibrosis, including with parametric T1 mapping and late gadolinium enhancement (LGE). In this study, we have used a dystrophic mouse model (D2.mdx) on the DBA/2J genetic background as a preclinical model of DMD.
Hypothesis: We hypothesize that CMR can detect worsening cardiac function and signs of fibrosis in the left ventricle of D2.mdx mice compared to wildtype (DBA) controls.
Methods: In a study group of 12 age-matched male mice (8 mdx, 4 DBA), CMR was performed with contrast to determine cardiac function and fibrotic developments in the heart.
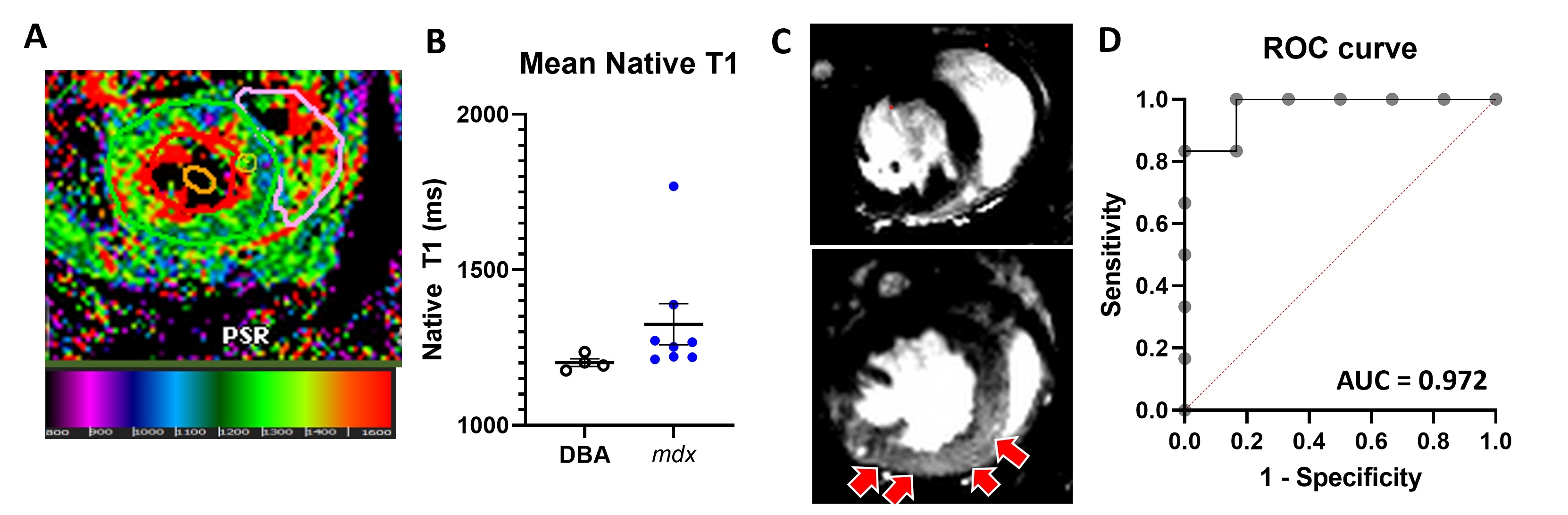
Results: D2.mdx mice displayed a higher left ventricular end-diastolic volume compared to DBA mice (32.8 ± 3.6 µL vs 27.4 ± 1.5 µL, p < 0.05). Global longitudinal strain was decreased in D2.mdx compared to DBA mice (-11.0 ± 3.5% vs -18.2 ± 2.2%, p < 0.05). Native T1 mapping (Fig 1A) showed significantly higher mean T1 times in D2.mdx mice compared to DBA controls (Fig 1B). LGE in the myocardium of the left ventricle was noted in 6 of 8 of the D2.mdx mice (Fig 1C), but not in DBA mice. Logistic regression modeling showed strong predictive value detecting LGE with increasing native T1 times (Fig 1D).
Conclusion: CMR can detect tissue changes in D2.mdx myocardium representing fibrotic changes. Native T1 may be useful in longitudinal studies of antifibrotic therapies in these models.
Hypothesis: We hypothesize that CMR can detect worsening cardiac function and signs of fibrosis in the left ventricle of D2.mdx mice compared to wildtype (DBA) controls.
Methods: In a study group of 12 age-matched male mice (8 mdx, 4 DBA), CMR was performed with contrast to determine cardiac function and fibrotic developments in the heart.
Results: D2.mdx mice displayed a higher left ventricular end-diastolic volume compared to DBA mice (32.8 ± 3.6 µL vs 27.4 ± 1.5 µL, p < 0.05). Global longitudinal strain was decreased in D2.mdx compared to DBA mice (-11.0 ± 3.5% vs -18.2 ± 2.2%, p < 0.05). Native T1 mapping (Fig 1A) showed significantly higher mean T1 times in D2.mdx mice compared to DBA controls (Fig 1B). LGE in the myocardium of the left ventricle was noted in 6 of 8 of the D2.mdx mice (Fig 1C), but not in DBA mice. Logistic regression modeling showed strong predictive value detecting LGE with increasing native T1 times (Fig 1D).
Conclusion: CMR can detect tissue changes in D2.mdx myocardium representing fibrotic changes. Native T1 may be useful in longitudinal studies of antifibrotic therapies in these models.
More abstracts on this topic:
Activation of the Histamine-3 Receptor Prevents Cardiac Fibrosis and Diastolic Dysfunction by Opposing a Profibrotic Cardiac Fibroblast Phenotype through Inhibition of cAMP Signaling
Connery Heather, Herrnreiter Anja, Campbell William, Widiapradja Alexander, Levick Scott
A Case of Caseous Mitral Annular Calcification and the Utility of Multimodality Cardiac ImagingNguyen Amanda, English Carter, Ghasemiesfe Ahmadreza, Venugopal Sandhya