Final ID: We133
In Silico Model of Cardiomyopathy Caused by a MYBPC3-induced HCM Mutation
Abstract Body: Introduction: Hypertrophic cardiomyopathy (HCM) is the most frequent hereditary cardiomyopathy and a leading cause of sudden cardiac death, particularly in adolescent. Despite recent improved prognosis and FDA approved drugs, current cardiac therapeutics cannot efficiently prevent remodeling across all HCM patient populations due to genetic variability. Therefore, we require a systems biology approach to understand how the complex cardiac signaling networks regulate cardiomyocyte shape and size due to an HCM mutation. This study leverages a computational systems approach to explore MYBPC3-induced HCM mutation regulation of cardiomyocyte hypertrophy.
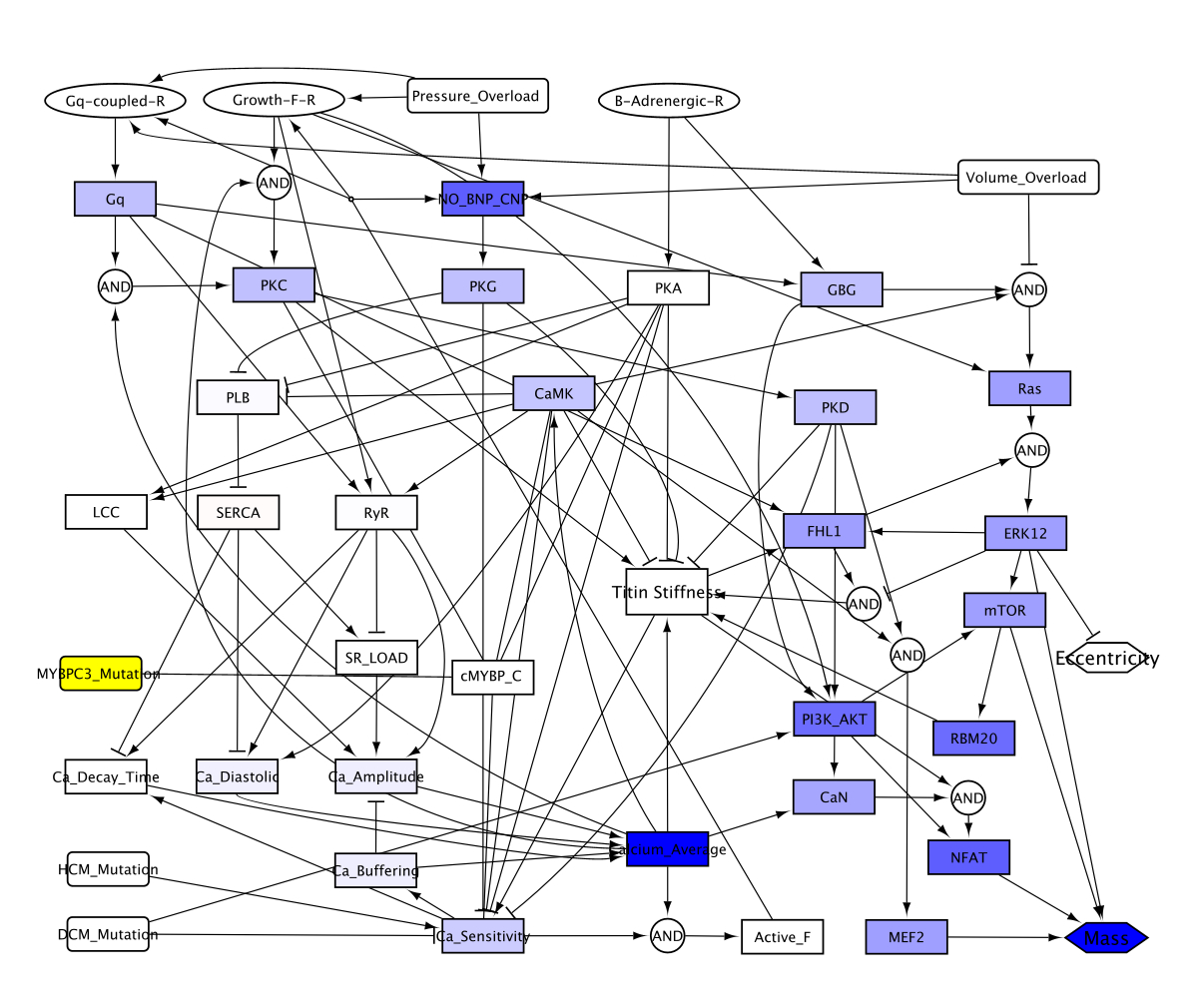
Method: For the signaling network model, we employed a logic-based differential equations model (NETFLUX) to construct a signaling network validated using in vitro data from published studies. This model captured signaling dynamics and cross talks between pathways governing cardiomyocyte size and shape. Additionally, a MYBPC3 HCM mutation is integrated in the model as an input providing a comparison between a control and simulated HCM responses. Sensitivity analyses are performed to investigate the most influential signaling pathways that mediate changes in mass.
Results: We created a MYBPC3-induced HCM model by integrating an additional cardiac myosin binding protein-C node to a previously validated inherited cardiomyopathy signaling network. This updated model predicted an increase in mass when the HCM mutation is simulated. The simulated inhibition of each node in the model revealed that inhibition of calcium pathway, growth factor receptor, and titin stiffness could mitigate the HCM phenotype by reducing mass. The sensitivity analysis results also suggested PI3K, Ras, and ERK1/2 pathways as potential mechanisms mediating the decrease in mass and served as potential therapeutic targets.
Discussion and Future Work: Our systems approach gave insights on the mechanisms governing the cardiomyocyte response to a MYBPC3-induced HCM mutation. This will be further validated via a MYBPC3-HCM model using human iPSC-CMs with MYBPC3-induced mutations. This will create a cell line that will provide genetically precise comparisons for identifying new molecular mechanisms of disease, thereby offering a strategy for advancing precision medicine.
Method: For the signaling network model, we employed a logic-based differential equations model (NETFLUX) to construct a signaling network validated using in vitro data from published studies. This model captured signaling dynamics and cross talks between pathways governing cardiomyocyte size and shape. Additionally, a MYBPC3 HCM mutation is integrated in the model as an input providing a comparison between a control and simulated HCM responses. Sensitivity analyses are performed to investigate the most influential signaling pathways that mediate changes in mass.
Results: We created a MYBPC3-induced HCM model by integrating an additional cardiac myosin binding protein-C node to a previously validated inherited cardiomyopathy signaling network. This updated model predicted an increase in mass when the HCM mutation is simulated. The simulated inhibition of each node in the model revealed that inhibition of calcium pathway, growth factor receptor, and titin stiffness could mitigate the HCM phenotype by reducing mass. The sensitivity analysis results also suggested PI3K, Ras, and ERK1/2 pathways as potential mechanisms mediating the decrease in mass and served as potential therapeutic targets.
Discussion and Future Work: Our systems approach gave insights on the mechanisms governing the cardiomyocyte response to a MYBPC3-induced HCM mutation. This will be further validated via a MYBPC3-HCM model using human iPSC-CMs with MYBPC3-induced mutations. This will create a cell line that will provide genetically precise comparisons for identifying new molecular mechanisms of disease, thereby offering a strategy for advancing precision medicine.
More abstracts on this topic:
Acute sleep deprivation induces cardiac remodeling via activation of AT1R/ERK/GSK-3β signaling
Luo Tao, Liu Haiqiong
An experimental and theoretical study on electrical brain stimulation for post Intracerebral Hemorrhagic stroke rehabilitation.Muguli Ananya, Luan Lan, Xie Chong, Britz Gavin, Aazhang Behnaam