Final ID: Tu002
Mesenchymal Stem Cell-Derived Extracellular Vesicles Mitigate Mitochondrial DNA-Induced Activation of Porcine Peripheral Blood Mononuclear Cells
Abstract Body: Objective: Systemic inflammation is a well-established component of post-cardiac arrest syndrome (PCAS), a condition responsible for significant morbidity and mortality in patients that are initially resuscitated from sudden cardiac arrest. Immune cell activation is pivotal in PCAS pathophysiology, with emerging evidence implicating mitochondrial DNA (mtDNA) as a potent pro-inflammatory stimulus in this context. Given the paucity of available interventions to inhibit mtDNA-induced immune cell activation, we investigated the therapeutic potential of mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) to modulate the inflammatory response of porcine peripheral blood mononuclear cells (PBMCs) activated by mtDNA in vitro.
Methods: Porcine bone marrow-derived mesenchymal stem cells (MSCs; P2) were cultured for 5 days and serum-starved for 3 days to collect MSC-EVs. These EVs were isolated from conditioned media (CM) using size exclusion chromatography and ultracentrifugation, then characterized by nanoparticle tracking analysis (ZetaView) and ExoCheck. Naïve PBMCs from healthy swine were cultured for 1 week before stimulation with lipofectamine 2000 (TR-control) or 2 µg/mL mtDNA+TR. MSC-EVs were added to activated PBMCs (2.5e8–2e9 EVs/well) and inflammatory surface marker expression (flow cytometry), inflammatory cytokine transcripts (qPCR) and release (ELISA), and reactive oxygen/nitrogen species (ROS/RNS) production were assessed 24 hours later.
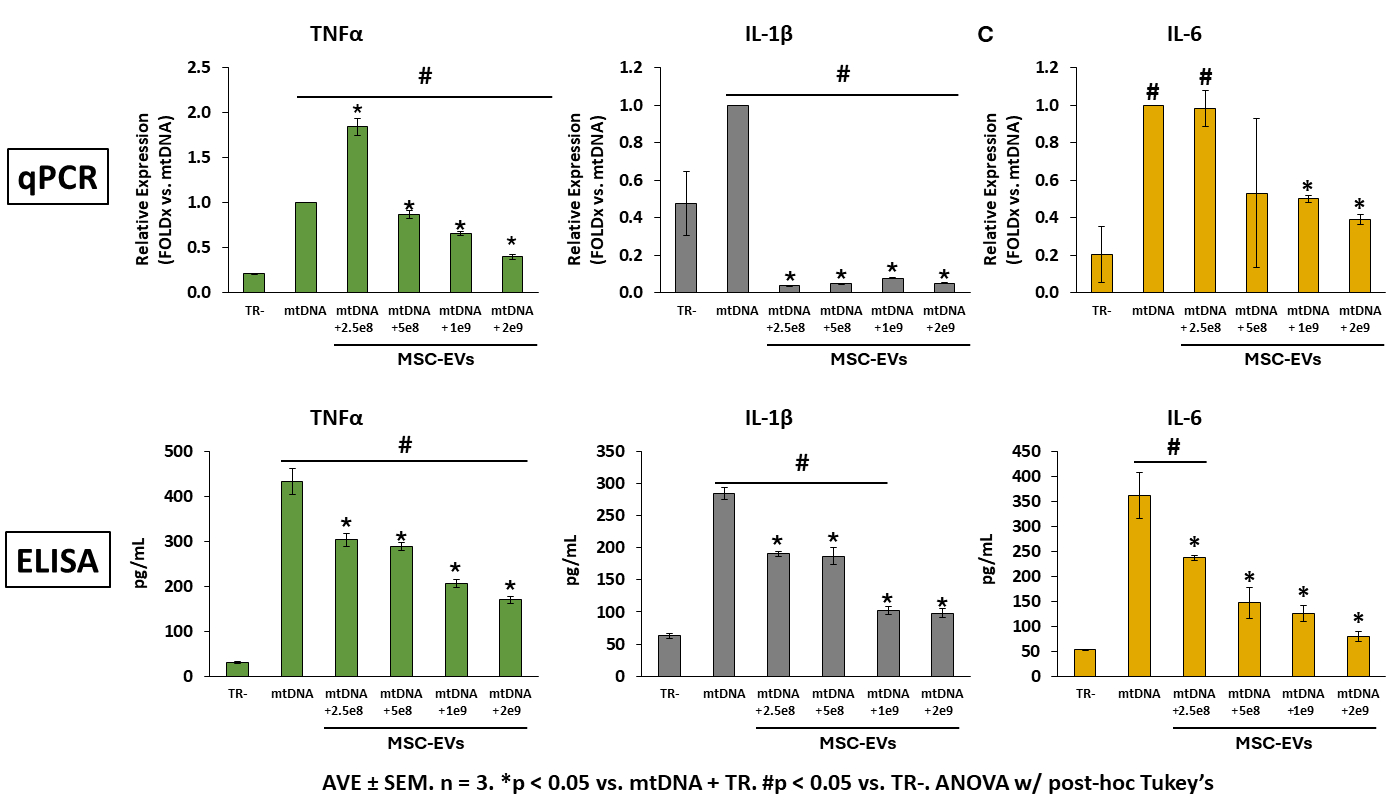
Results: mtDNA-stimulated PBMC populations exhibited altered surface marker expression consistent with enrichment of inflammatory granulocytes (CD172+CD163-), macrophages (CD14+CD163+), and adhesion molecules (CD172+) compared to TR-control (all p<0.05). Addition of MSC-EVs significantly reduced the number of all inflammatory cell populations, with the most prominent effects achieved at the highest EV dose (2e9 EVs/well). Likewise, MSC-EVs significantly attenuated mtDNA-activated PBMC expression and secretion of TNFα, IL-1β, and IL-6 (Figure) and decreased ROS/RNS production in a dose-dependent manner.
Conclusion: MSC-EVs attenuate mtDNA-induced activation of PBMCs in a dose-dependent manner, as determined by reductions in pro-inflammatory surface marker expression, inflammatory cytokine gene expression and secretion, and ROS/RNS production. These findings indicate that MSC-EVs may be a viable therapeutic for the inhibition of mtDNA-mediated immune activation in PCAS.
Methods: Porcine bone marrow-derived mesenchymal stem cells (MSCs; P2) were cultured for 5 days and serum-starved for 3 days to collect MSC-EVs. These EVs were isolated from conditioned media (CM) using size exclusion chromatography and ultracentrifugation, then characterized by nanoparticle tracking analysis (ZetaView) and ExoCheck. Naïve PBMCs from healthy swine were cultured for 1 week before stimulation with lipofectamine 2000 (TR-control) or 2 µg/mL mtDNA+TR. MSC-EVs were added to activated PBMCs (2.5e8–2e9 EVs/well) and inflammatory surface marker expression (flow cytometry), inflammatory cytokine transcripts (qPCR) and release (ELISA), and reactive oxygen/nitrogen species (ROS/RNS) production were assessed 24 hours later.
Results: mtDNA-stimulated PBMC populations exhibited altered surface marker expression consistent with enrichment of inflammatory granulocytes (CD172+CD163-), macrophages (CD14+CD163+), and adhesion molecules (CD172+) compared to TR-control (all p<0.05). Addition of MSC-EVs significantly reduced the number of all inflammatory cell populations, with the most prominent effects achieved at the highest EV dose (2e9 EVs/well). Likewise, MSC-EVs significantly attenuated mtDNA-activated PBMC expression and secretion of TNFα, IL-1β, and IL-6 (Figure) and decreased ROS/RNS production in a dose-dependent manner.
Conclusion: MSC-EVs attenuate mtDNA-induced activation of PBMCs in a dose-dependent manner, as determined by reductions in pro-inflammatory surface marker expression, inflammatory cytokine gene expression and secretion, and ROS/RNS production. These findings indicate that MSC-EVs may be a viable therapeutic for the inhibition of mtDNA-mediated immune activation in PCAS.
More abstracts on this topic:
A Novel Role for Lipoprotein(a) in Potentiating Neutrophil Extracellular Trap Formation
Mouawad Sahar, Boffa Michael, Koschinsky Marlys
Aging-Associated Protein Medin Induces Human Coronary Artery Endothelial Proinflammatory and Prothrombotic ActivationKaramanova Nina, Morrow Kaleb, Maerivoet Alana, Madine Jillian, Li Ming, Migrino Raymond