Final ID:
Evolocumab and Saphenous Vein Graft Patency Following Coronary Artery Bypass Graft (CABG) Surgery in People with and without Diabetes: A Pre-Specified Analysis of the Randomized Placebo Controlled NEWTON-CABG CardioLink-5 Trial
Saphenous vein graft (SVG) failure is a critical limitation for the long-term success of CABG surgery, with up to 30% of SVGs failing by 2 years. The NEWTON-CABG CardioLink-5 trial evaluated the effect of early evolocumab initiation on SVG patency 2 years post-surgery. Whether diabetes, a key driver of atherothrombosis, and a major determinant in surgical vs. percutaneous revascularization decision making, may modulate SVG disease progression is poorly defined. We report a pre-specified subgroup analysis evaluating SVG outcomes in patients with vs. without diabetes.
Methods:
NEWTON-CABG (NCT03900026) was a multicenter, randomized, double-blind, placebo-controlled trial that enrolled patients within 3–21 days post-CABG surgery (≥2 SVGs) who were on moderate- to high-intensity statin therapy. There was no pre-specified LDL-C entry criterion. Participants were randomized 1:1 to receive evolocumab 140 mg or placebo every 2 weeks for 2 years. The primary endpoint was SVG disease rate (≥50% stenosis in any SVG by CCTA or clinically indicated invasive angiography) at 2 years. Key secondary endpoints included total occlusion rates and the proportion of patients with ≥1 occluded SVG. This pre-specified analysis evaluated the primary and secondary outcomes as well as comparative efficacy of evolocumab vs. placebo by baseline diabetes status.
Results:
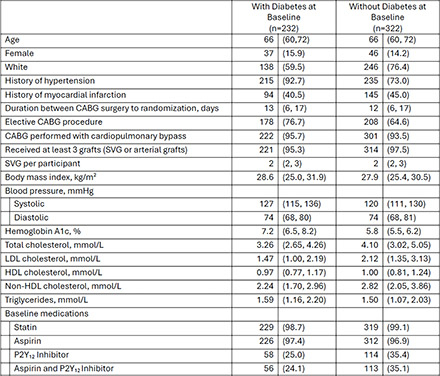
Of the 782 patients (median age 66 years, ~15% women) enrolled across 4 countries, ~42% had diabetes at baseline. Key baseline characteristics are shown in the Table. The median time from CABG surgery to randomization was 13 days. Full results of the primary endpoint are embargoed until a Hot Line presentation at ESC 2025. The proposed AHA late-breaker will present new data on (a) SVG failure rates by baseline diabetes status and (b) the impact of evolocumab vs. placebo on various endpoints in patients with and without diabetes including subgroup-specific rates of ≥50% SVG stenosis, total occlusions, patient-level graft failure, a composite win ratio, and SVG plaque volume.
Conclusions:
NEWTON-CABG is the first randomized trial to assess the effect of intensive LDL-C lowering with a PCSK9 inhibitor initiated early after CABG on SVG patency. This pre-specified analysis will provide pivotal insights into whether diabetes is an independent risk factor for SVG failure and whether evolocumab modifies this risk. These findings will have important implications for the large cohort of post-CABG patients with diabetes.
- Leiter, Lawrence ( ST MICHAELS HOSPITAL , Toronto , Ontario , Canada )
- Thorpe, Kevin ( University of Toronto , Toronto , Ontario , Canada )
- Saha, Tarit ( KINGSTON HEALTH SCIENCES CENTER , KINGSTON , Ontario , Canada )
- Whitlock, Richard ( McMaster University , Hamilton , Ontario , Canada )
- Yanagawa, Bobby ( St Michaels Hospital , Toronto , Ontario , Canada )
- Merkely, Béla ( Semmelweis University , Budapest , Hungary )
- Juni, Peter ( Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU) , Oxford , United Kingdom )
- Koren, Michael ( Jacksonville Ctr for Clinical Res , Jacksonville , Florida , United States )
- Nicholls, Stephen ( Victorian Heart Hospital , Clayton , Victoria , Australia )
- Bhatt, Deepak ( Mount Sinai Fuster Heart Hospital , Scarsdale , New York , United States )
- Mazer, David ( St Michaels Hospital, Toronto , Toronto , Ontario , Canada )
- Verma, Subodh ( ST MICHAELS HOSPITAL , Toronto , Ontario , Canada )
- Teoh, Hwee ( ST MICHAELS HOSPITAL , Toronto , Ontario , Canada )
- Mancini, G B John ( University of British Columbia , West Vancouver , British Columbia , Canada )
- Szarek, Michael ( UNIVERSITY OF COLORADO , Brooklyn , New York , United States )
- Quan, Adrian ( ST. MICHAEL'S HOSPITAL , Toronto , Ontario , Canada )
- Elituv, Randi ( North York Diagnostic and Cardiac Centre , Canada , Ontario , Canada )
- Verma, Meena ( Royal College of Surgeons in Ireland , Dublin , Ireland )
- Misner, Elizabeth ( ST MICHAELS HOSPITAL , Toronto , Ontario , Canada )
Meeting Info:
Session Info:
Lipid Therapies: Translation to Implementation
Monday, 11/10/2025 , 03:15PM - 04:30PM
Featured Science
More abstracts on this topic:
A Longitudinal 20-year Analysis Indicates Acceleration of Cardiometabolic Comorbidities on Dementia Risk
Lihua Huang, Danish Muhammad, Auyeung Tw, Jenny Lee, Kwok Timothy, Abrigo Jill, Wei Yingying, Lo Cecilia, Fung Erik
Activated CD8+HLA-DR+ T Cells as Immune Biomarkers of Metabolic Dysfunction and Cardiovascular Risk in PrediabetesAlrashed Fatema, Alsaeed Halemah, Alturaiki Wael, Akhter Nadeem, Alosaimi Bandar, Almutairi Saeedah, Mubarak Ayman, Al-mulla Fahd, Ahmad Rasheed
More abstracts from these authors:
Hibino Makoto, Teoh Hwee, Verma Subodh, Quan Adrian, Puar Pankaj, Akhavein Farhad, Wang Chao-hung, Yan Andrew, Connelly Kim, Mazer David
A Phase 2 Multicenter, Randomized, Double-Blind, Placebo-Controlled Study of Monthly or Quarterly Subcutaneous Administration of the Interleukin-6 Inhibitor Pacibekitug in Patients With Elevated High-Sensitivity C-Reactive Protein and Chronic Kidney Disease: 90-Day Analyses from TRANQUILITYPergola Pablo, Szarek Michael, Zayed Hany, Hemani Famina, Degoma Emil, Andorfer Cathy, Walsh John, Ridker Paul, Bhatt Deepak