Final ID: MDP312
Prognostic Value of NT-proBNP and the H2FPEF Score on Empagliflozin-associated Left Ventricular Remodeling: Insights from the EMPA-HEART and EMPA-HEART 2 CardioLink Trial
Abstract Body (Do not enter title and authors here): Background: NT-proBNP is a prognostic and diagnostic marker for heart failure while the H2FPEF score is used to aid in diagnosing heart failure with preserved ejection fraction.
Aims: These post hoc analyses of the randomized controlled EMPA-HEART CardioLink-6 and EMPA-HEART 2 CardioLink-7 trials examined how baseline NT-proBNP and the H2FPEF score influenced empagliflozin-associated left ventricular (LV) remodeling in people with either type 2 diabetes and coronary artery disease or without diabetes but with cardiovascular risk factors.
Methods: A total of 242 participants were assigned to either empagliflozin 10 mg QD (n=118) or placebo (n=124) for 6 months. NT-proBNP was measured and cardiac MRI performed at baseline and end-of-study. Two independent analyses were performed. One divided the participants into those with baseline NT-proBNP < and ≧125 pg/mL and the other into those with H2FPEF score ≦2 and ≧3. The relationships between empagliflozin-associated LV mass and functional changes with baseline NT-proBNP and H2FPEF scores were assessed with linear models that adjusted for baseline differences (ANCOVA).
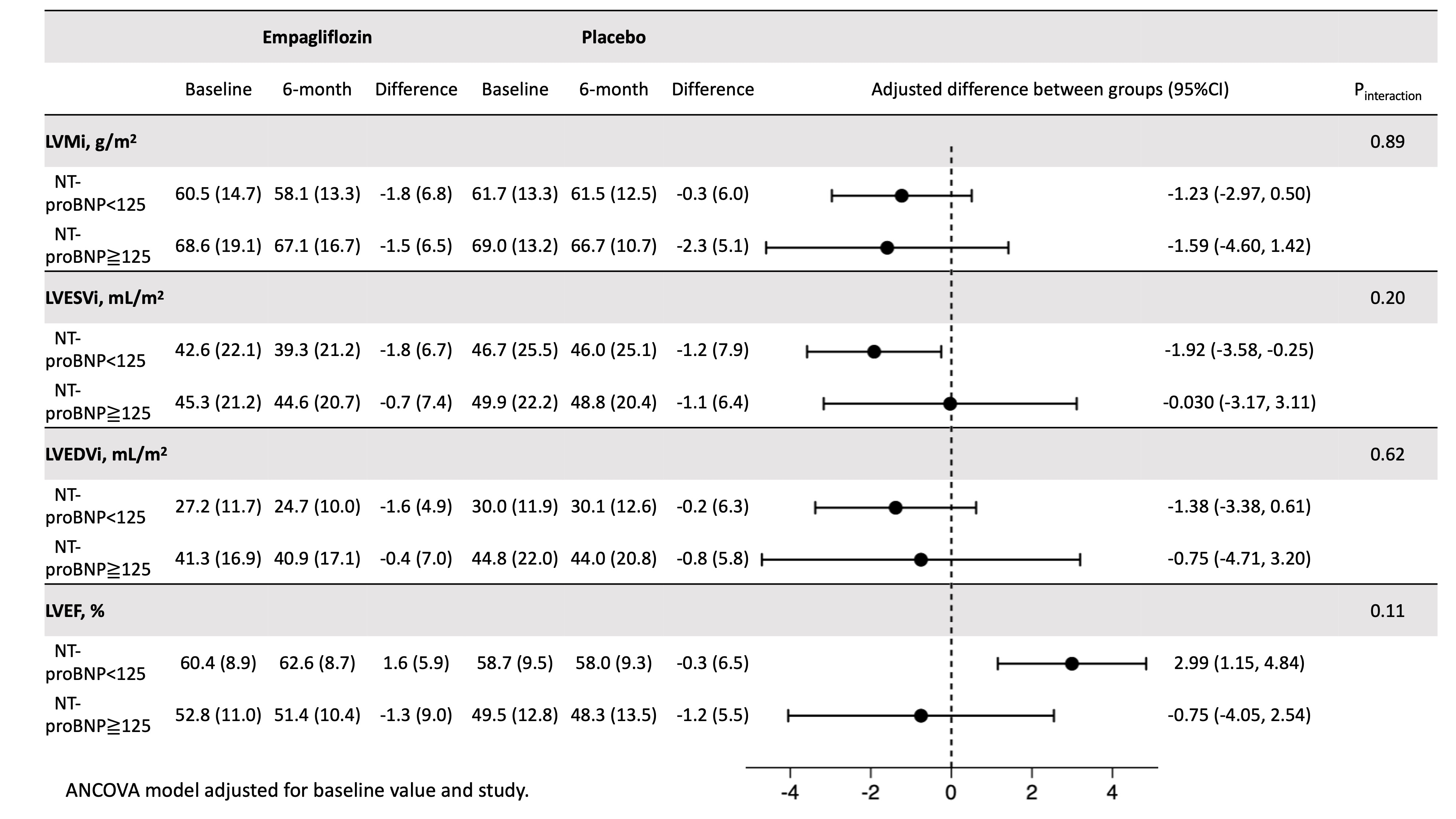
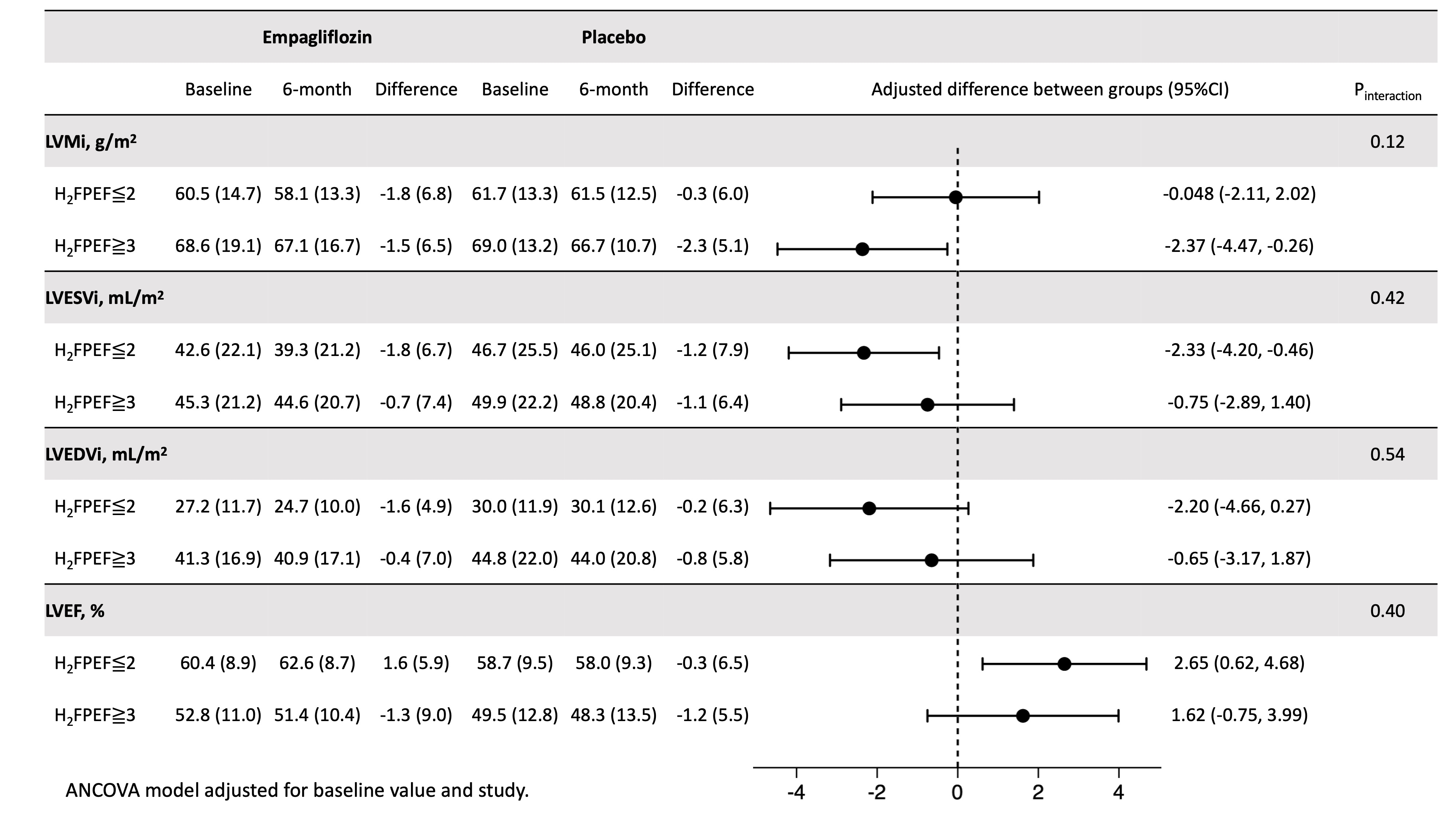
Results: Mean age of the pooled cohort was 60.6 years; 14.0% were females, 37.2% had type 2 diabetes. Mean (SD) LV ejection fraction (LVEF) was 58.0 (10.2) %; median (IQR) NT-proBNP 77.5 (33, 164) pg/mL; mean (SD) H2FPEF score 2.9 (1.4). Empagliflozin (vs placebo) did not significantly alter LV mass index (mean [SD] -1.35 g/m2 [0.75]; P=0.07) and LV end-systolic volume index (LVESVi; mean [SD] -1.37 mL/m2 [0.74]; P=0.07) but did improve LVEF (mean [SD] 2.08% [0.82]; P=0.01). The effect of empagliflozin based on the subgroups of baseline NT-proBNP and H2FPEF scores are shown in Figures 1 and 2, respectively.
Conclusions:
These post hoc analyses suggest that lower baseline NT-proBNP levels and H2FPEF scores may be associated with more favourable reverse LV remodelling by empagliflozin and should be validated in larger studies.
Aims: These post hoc analyses of the randomized controlled EMPA-HEART CardioLink-6 and EMPA-HEART 2 CardioLink-7 trials examined how baseline NT-proBNP and the H2FPEF score influenced empagliflozin-associated left ventricular (LV) remodeling in people with either type 2 diabetes and coronary artery disease or without diabetes but with cardiovascular risk factors.
Methods: A total of 242 participants were assigned to either empagliflozin 10 mg QD (n=118) or placebo (n=124) for 6 months. NT-proBNP was measured and cardiac MRI performed at baseline and end-of-study. Two independent analyses were performed. One divided the participants into those with baseline NT-proBNP < and ≧125 pg/mL and the other into those with H2FPEF score ≦2 and ≧3. The relationships between empagliflozin-associated LV mass and functional changes with baseline NT-proBNP and H2FPEF scores were assessed with linear models that adjusted for baseline differences (ANCOVA).
Results: Mean age of the pooled cohort was 60.6 years; 14.0% were females, 37.2% had type 2 diabetes. Mean (SD) LV ejection fraction (LVEF) was 58.0 (10.2) %; median (IQR) NT-proBNP 77.5 (33, 164) pg/mL; mean (SD) H2FPEF score 2.9 (1.4). Empagliflozin (vs placebo) did not significantly alter LV mass index (mean [SD] -1.35 g/m2 [0.75]; P=0.07) and LV end-systolic volume index (LVESVi; mean [SD] -1.37 mL/m2 [0.74]; P=0.07) but did improve LVEF (mean [SD] 2.08% [0.82]; P=0.01). The effect of empagliflozin based on the subgroups of baseline NT-proBNP and H2FPEF scores are shown in Figures 1 and 2, respectively.
Conclusions:
These post hoc analyses suggest that lower baseline NT-proBNP levels and H2FPEF scores may be associated with more favourable reverse LV remodelling by empagliflozin and should be validated in larger studies.
More abstracts on this topic:
Additive effect of nighttime blood pressure on left ventricular strain in patients with primary aldosteronism evaluated via cardiac magnetic resonance tissue tracking
Wan Jindong, Liu Sen, Yang Yi, Wang Xinquan, Wang Dan, Zhou Peng, Wang Peijian
A Case of Concomitant Wild-Type Transthyretin and Systemic Light Chain Amyloidosis Involving Separate OrgansChiu Leonard, Afrough Aimaz, Nadeem Urooba, Jebakumar Deborah, Grodin Justin