Final ID:
Primary results from the phase 2 randomized, placebo controlled, blinded trial of the monoclonal antibody coramitug in transthyretin amyloid cardiomyopathy (ATTR-CM)
Methods: This phase 2, double blind, placebo-controlled trial randomized participants with heart failure (NYHA Class II or III) due to ATTR-CM to receive intravenous infusions every 4 weeks of either coramitug at two dosages (10 mg/kg or 60 mg/kg) or placebo in a 1:1:1 ratio for 52 weeks. The primary endpoints were the change from baseline to week 52 in the six-minute walk test (6MWT) and NT-proBNP. Safety was assessed for up to 64 weeks by treatment-emergent adverse events, all-cause mortality, number of cardiovascular (CV) events comprising CV-related hospitalization or urgent heart failure visits.
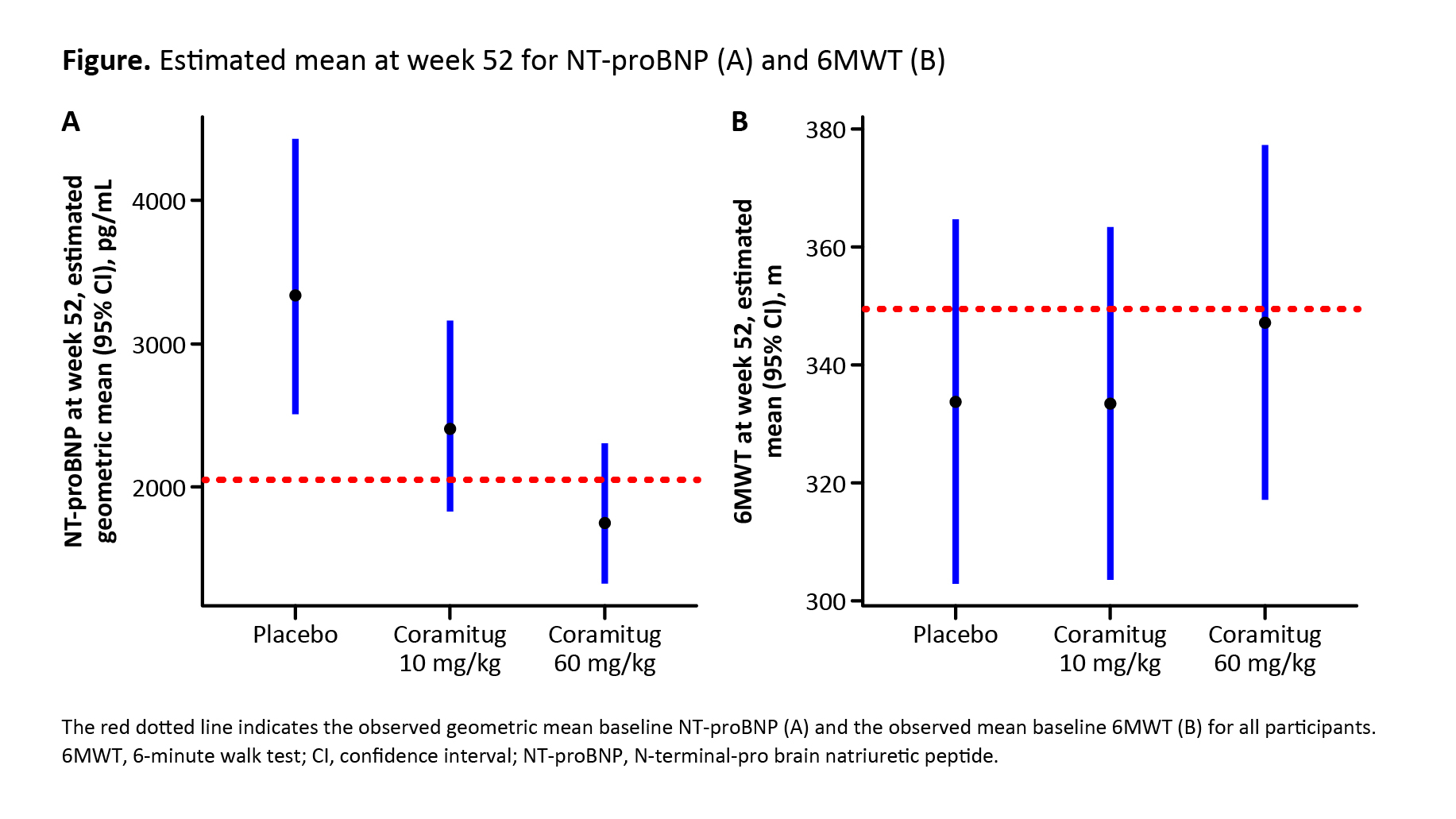
Results: A total of 104 participants were randomized and dosed: 34 to coramitug 10 mg/kg, 35 to coramitug 60 mg/kg, 35 to placebo. Mean age was 76 years; 93% were men; 84% and 16% were in NYHA class II and III, respectively; and 62%, 21%, 14% and 3% were NAC stage 1–4, respectively. Overall, 13% had variant type ATTR-CM; median NT-proBNP was 1985 pg/mL (range: 371 to 12,890 pg/mL); and 90% of participants were on disease-modifying therapy. Baseline characteristics were generally well balanced across the treatment arms. From baseline to week 52, coramitug 60 mg/kg was associated with a significant reduction in NT-proBNP compared with placebo (–48%; 95% CI: –65% to –22%; p=0.0017), whereas coramitug 10 mg/kg was not associated with a significant reduction (–28%; 95% CI: –51% to +7%; p=0.1043) (see also Figure A). There was no statistically significant difference in the change in 6MWT from baseline to week 52 with either dose compared with placebo (Figure B). Coramitug was well tolerated. Treatment-emergent adverse events were numerically less frequent with coramitug 60 mg/kg (216 events) and coramitug 10 mg/kg (257 events), compared with placebo (311 events).
Conclusions: In the first phase 2 trial of an antibody targeting misfolded transthyretin, treatment with the humanized antibody coramitug 60 mg/kg in ATTR-CM was well tolerated and resulted in a statistically significant reduction in NT-proBNP, a validated marker of disease progression.
- Fontana, Marianna ( UCL , London , United Kingdom )
- Kar, Soumitra ( Novo Nordisk Service Centre Pvt Ltd , Bangalore , India )
- Revanna, Manjunatha ( Novo Nordisk A/S , Søborg , Denmark )
- Sarswat, Nitasha ( University of Chicago , Chicago , Illinois , United States )
- Tsujita, Kenichi ( Kumamoto University , Kumamoto , Japan )
- Maurer, Mathew ( Columbia University , New York , New York , United States )
- Garcia-pavia, Pablo ( Hospital Puerta de Hierro , Majadahonda , Spain )
- Grogan, Martha ( Mayo Clinic , Rochester , Minnesota , United States )
- Shah, Sanjiv ( Northwestern University , Chicago , Illinois , United States )
- Engelmann, Mads ( Novo Nordisk A/S , Søborg , Denmark )
- Hovingh, G. Kees ( Novo Nordisk A/S , Søborg , Denmark )
- Kristen, Arnt ( Medical University of Heidelberg , Heidelberg , Germany )
- Lim-watson, Michelle ( Novo Nordisk US R&D , Lexington , Massachusetts , United States )
- Malling, Brian ( Novo Nordisk US R&D , Lexington , Massachusetts , United States )
Meeting Info:
Session Info:
Rewriting the Code for Cardiac Amyloid: Novel Identification, Treatment, and Cure
Monday, 11/10/2025 , 01:30PM - 02:45PM
Featured Science
More abstracts on this topic:
Keshvani Neil, Wang Thomas, Pandey Ambarish, Coellar Juan David, Rizvi Syed Kazim, Jain Anand, Bustillo-rubio M. Karina, Segar Matthew, Lokesh Nidhish, Miller James, Yates Sean
A Case of Clozapine-Induced Myocarditis: An Under-described Side EffectIbrahim Rand, Clearo Kellie
More abstracts from these authors:
Judge Daniel, Masri Ahmad, Obici Laura, Poulsen Steen, Sarswat Nitasha, Shah Keyur, Soman Prem, Cao Xiaofan, Wang Kevin, Pecoraro Maria, Tamby Jean-francois, Gillmore Julian, Katz Leonid, Fox Jonathan, Maurer Mathew, Alexander Kevin, Ambardekar Amrut, Cappelli Francesco, Fontana Marianna, Garcia-pavia Pablo, Grogan Martha, Hanna Mazen
Acoramidis Reduces All-Cause Mortality and Cardiovascular-Related Hospitalizations Through Month 42 in Transthyretin Amyloid Cardiomyopathy Across All Pre-specified Patient SubgroupsStern Lily, Fine Nowell, Maurer Mathew, Grogan Martha, Ambardekar Amrut, Grodin Justin, Soman Prem, Garcia-pavia Pablo, Chen Chris, Siddhanti Suresh, Tamby Jean-francois, Fox Jonathan