Final ID:
SHR-1918, an Angiopoietin-Like 3 Antibody, in Patients With Suboptimally Controlled Hyperlipidemia
Abstract Body (Do not enter title and authors here): Introduction
Low-density lipoprotein cholesterol (LDL-C) and triglyceride (TG) are established risk factors for atherosclerotic cardiovascular diseases. SHR-1918 is a long-acting angiopoietin-like 3 (ANGPTL3) monoclonal antibody designed to lower LDL-C and TG levels through the regulation of ANGPTL3. This study assessed the efficacy and safety of SHR-1918 subcutaneous injection in patients with suboptimally controlled hyperlipidemia.
Methods
In this randomized, double-blind, placebo-controlled phase 2 trial, hyperlipidemic patients who did not achieve LDL-C targets after 4-8 weeks of statin run-in were randomized (2:1) to receive subcutaneous SHR-1918 (600 mg or 1200 mg Q12W) or placebo for 24 weeks, followed by 8 weeks of safety observation. Background lipid-lowering therapies remained stable during the treatment period. The primary and key secondary endpoints were percentage change in LDL-C and TG from baseline to Week 24.
Results
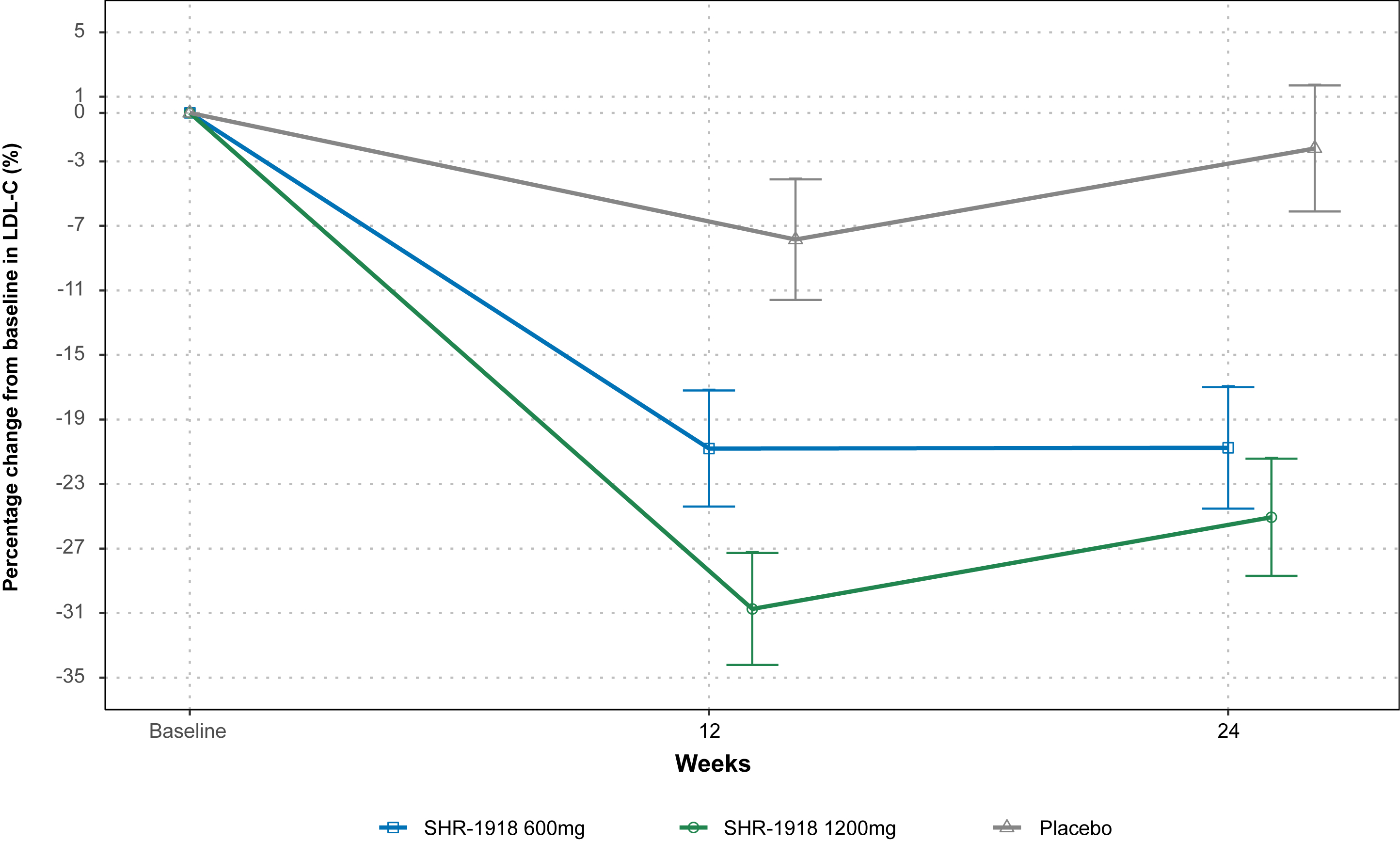
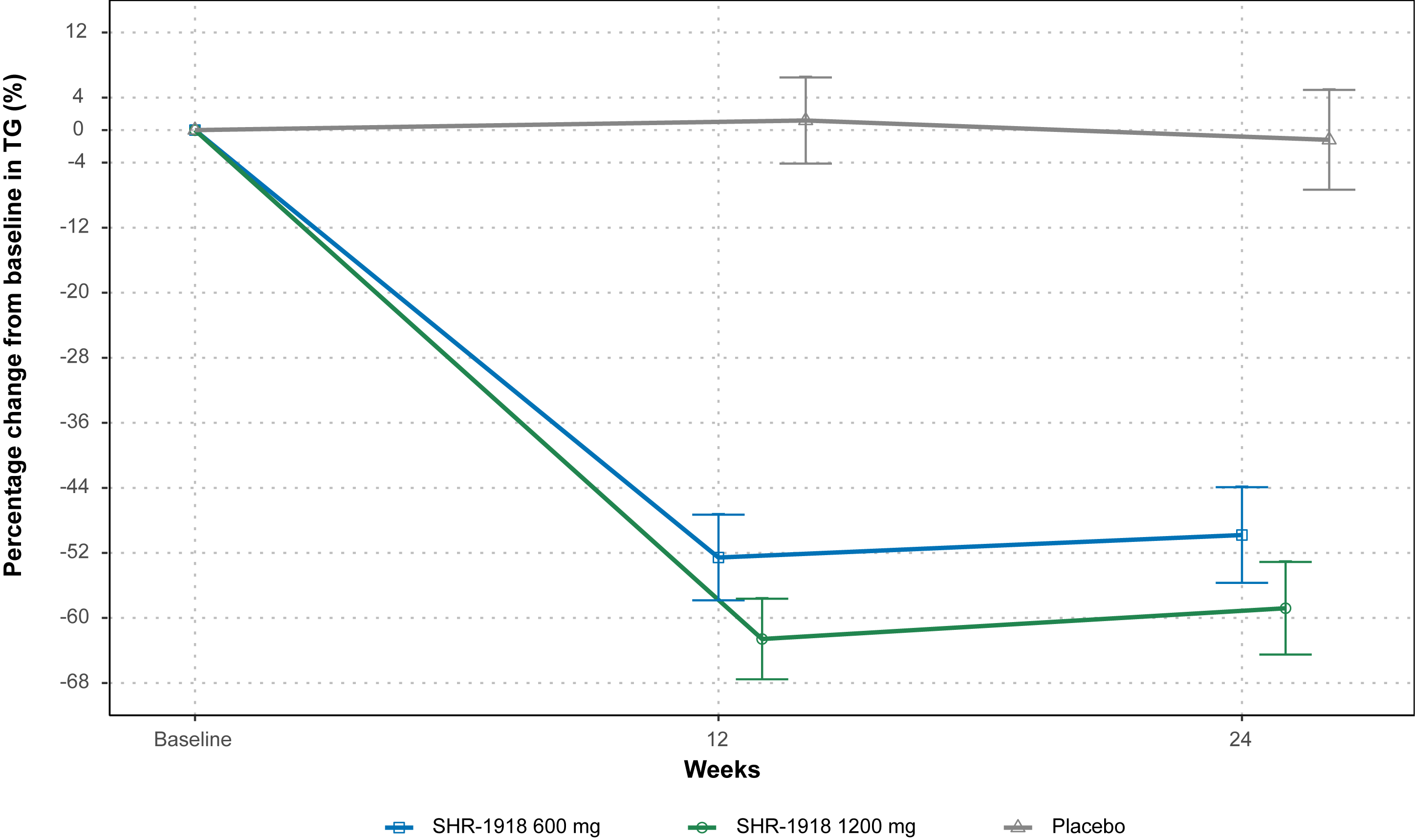
44 patients were randomized and treated (SHR-1918 600 mg, n=14; SHR-1918 1200 mg, n=16; placebo, n=14). Baseline LDL-C and TG were generally balanced. At Week 24, the least squares mean percentage change in LDL-C was -20.8%, -25.1%, and -2.2% in the SHR-1918 600 mg, SHR-1918 1200 mg, and placebo groups, respectively (Figure 1). The between group difference (SHR-1918 vs placebo) was -18.6% (95% CI, -29.5 to -7.6; P=0.0015) for 600 mg and -22.9% (95% CI, -33.7 to -12.1%; P=0.0001) for 1200 mg. TG decreased by 49.8% and 58.8% with SHR-1918 600 mg and 1200 mg, compared to 1.2% with placebo (Figure 2). The between-group difference in TG change from baseline was -48.6% (95% CI, -65.8 to -31.4; P<0.0001) for 600 mg and -57.6% (95% CI, -74.6 to -40.6; P<0.0001) for 1200 mg. Significant reductions were also observed in non-high-density lipoprotein cholesterol, total cholesterol, and other lipids after 24 weeks of SHR-1918 treatment.
Treatment-emergent adverse events (TEAEs) were reported by 22 (73.3%) SHR-1918-treated patients and 11 (78.6%) placebo-treated patients, all of which were mild or moderate in severity. 8 (26.7%) patients had SHR-1918-related TEAEs. There were no TEAEs leading to treatment discontinuation or death during the study.
Conclusion
SHR-1918 further reduced LDL-C and TG levels in hyperlipidemic patients on standard lipid-lowering therapies, providing a novel and safe treatment option for hyperlipidemia.
Low-density lipoprotein cholesterol (LDL-C) and triglyceride (TG) are established risk factors for atherosclerotic cardiovascular diseases. SHR-1918 is a long-acting angiopoietin-like 3 (ANGPTL3) monoclonal antibody designed to lower LDL-C and TG levels through the regulation of ANGPTL3. This study assessed the efficacy and safety of SHR-1918 subcutaneous injection in patients with suboptimally controlled hyperlipidemia.
Methods
In this randomized, double-blind, placebo-controlled phase 2 trial, hyperlipidemic patients who did not achieve LDL-C targets after 4-8 weeks of statin run-in were randomized (2:1) to receive subcutaneous SHR-1918 (600 mg or 1200 mg Q12W) or placebo for 24 weeks, followed by 8 weeks of safety observation. Background lipid-lowering therapies remained stable during the treatment period. The primary and key secondary endpoints were percentage change in LDL-C and TG from baseline to Week 24.
Results
44 patients were randomized and treated (SHR-1918 600 mg, n=14; SHR-1918 1200 mg, n=16; placebo, n=14). Baseline LDL-C and TG were generally balanced. At Week 24, the least squares mean percentage change in LDL-C was -20.8%, -25.1%, and -2.2% in the SHR-1918 600 mg, SHR-1918 1200 mg, and placebo groups, respectively (Figure 1). The between group difference (SHR-1918 vs placebo) was -18.6% (95% CI, -29.5 to -7.6; P=0.0015) for 600 mg and -22.9% (95% CI, -33.7 to -12.1%; P=0.0001) for 1200 mg. TG decreased by 49.8% and 58.8% with SHR-1918 600 mg and 1200 mg, compared to 1.2% with placebo (Figure 2). The between-group difference in TG change from baseline was -48.6% (95% CI, -65.8 to -31.4; P<0.0001) for 600 mg and -57.6% (95% CI, -74.6 to -40.6; P<0.0001) for 1200 mg. Significant reductions were also observed in non-high-density lipoprotein cholesterol, total cholesterol, and other lipids after 24 weeks of SHR-1918 treatment.
Treatment-emergent adverse events (TEAEs) were reported by 22 (73.3%) SHR-1918-treated patients and 11 (78.6%) placebo-treated patients, all of which were mild or moderate in severity. 8 (26.7%) patients had SHR-1918-related TEAEs. There were no TEAEs leading to treatment discontinuation or death during the study.
Conclusion
SHR-1918 further reduced LDL-C and TG levels in hyperlipidemic patients on standard lipid-lowering therapies, providing a novel and safe treatment option for hyperlipidemia.
More abstracts on this topic:
A Randomized Clinical Trial for Asymptomatic Elevated Blood Pressure in Patients Discharged from Emergency Department
Prendergast Heather, Khosla Shaveta, Kitsiou Spyros, Petzel Gimbar Renee, Freels Sally, Sanders Anissa, Daviglus Martha, Carter Barry, Del Rios Marina, Heinert Sara
A Focus for Improvement - Factors for Lab Adherence in a Pediatric Preventive Cardiology ProgramHolsinger Hunter, Porterfield Ronna, Taylor Makenna, Dresbach Bethany, Seipel Brittany, Igwe Chukwuemeka, Alvarado Chance, Tran Andrew