Final ID:
The Foot-PAD Trial: A Double-Blind, Randomized Placebo-Controlled Trial to determine the effect of a 12-Week Program of Footplate Neuromuscular Electrical Stimulation on Walking Capacity in Patients with Peripheral Artery Disease
Abstract Body (Do not enter title and authors here): Background: Patients with peripheral artery disease (PAD) experience leg muscle pain that limits walking and the ability to adhere to exercise therapy. Neuromuscular electrical stimulation (NMES) induces exercise-like muscle contractions and can be performed without pain. Hypothesis and Purpose: We hypothesised that a 12-week program of NMES would improve walking capacity compared with placebo-control in patients with PAD.
Study Design and Methods: Single-centre, parallel-group, double-blinded, randomized placebo-controlled trial. Participants were randomized to a 12-week program of NMES or sham placebo-control (CON). This was followed by a 6-week off-intervention follow-up. Outcomes were assessed at baseline (week 0), midway (week 6) and at the end of the intervention period (week 12), and again at the end of the follow-up phase (week 18). Sample Size: 180 patients (n=90 per group) from public hospital, private hospital and community clinic recruitment sites. Population Studied: Adults (mean age: 72 ± 9 y; ABI: 0.69 ± 0.20) with a diagnosis of PAD who experience leg pain when walking (intermittent claudication). Interventions: Participants used a footplate-NMES device (Revitive Medic Coach) for 2x30 min periods each day to deliver stimulation sufficient to induce contraction of the leg muscles. Control participants used a sham device that delivers a low intensity stimulation that does not induce muscle contraction. Primary End Point: Maximum distance during the six-minute walk test (6MWT) at week 12. Secondary End Points: Pain-free distance during the 6MWT, maximum and pain-free walking time during a graded treadmill test, self-reported walking impairment questionnaire (WIQ), disease-specific QOL assessed using the intermittent claudication questionnaire (ICQ), and accelerometer derived physical activity levels. Power Calculation: At an alpha-level of 0.05 and a power of 0.80 the trial was powered to detect a 30 m change (effect size 0.33) in 6MWT distance in the NMES group compared with CON.
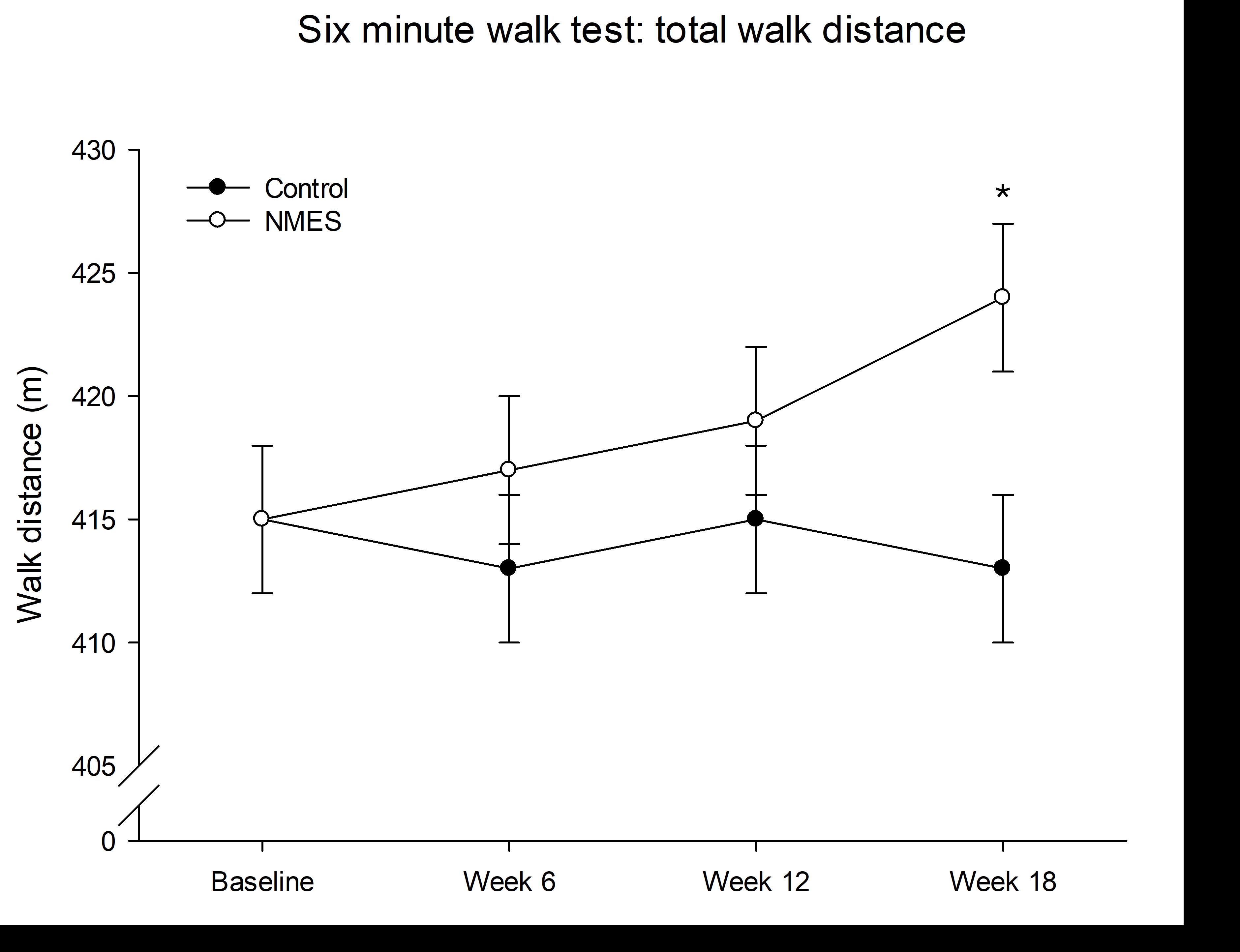
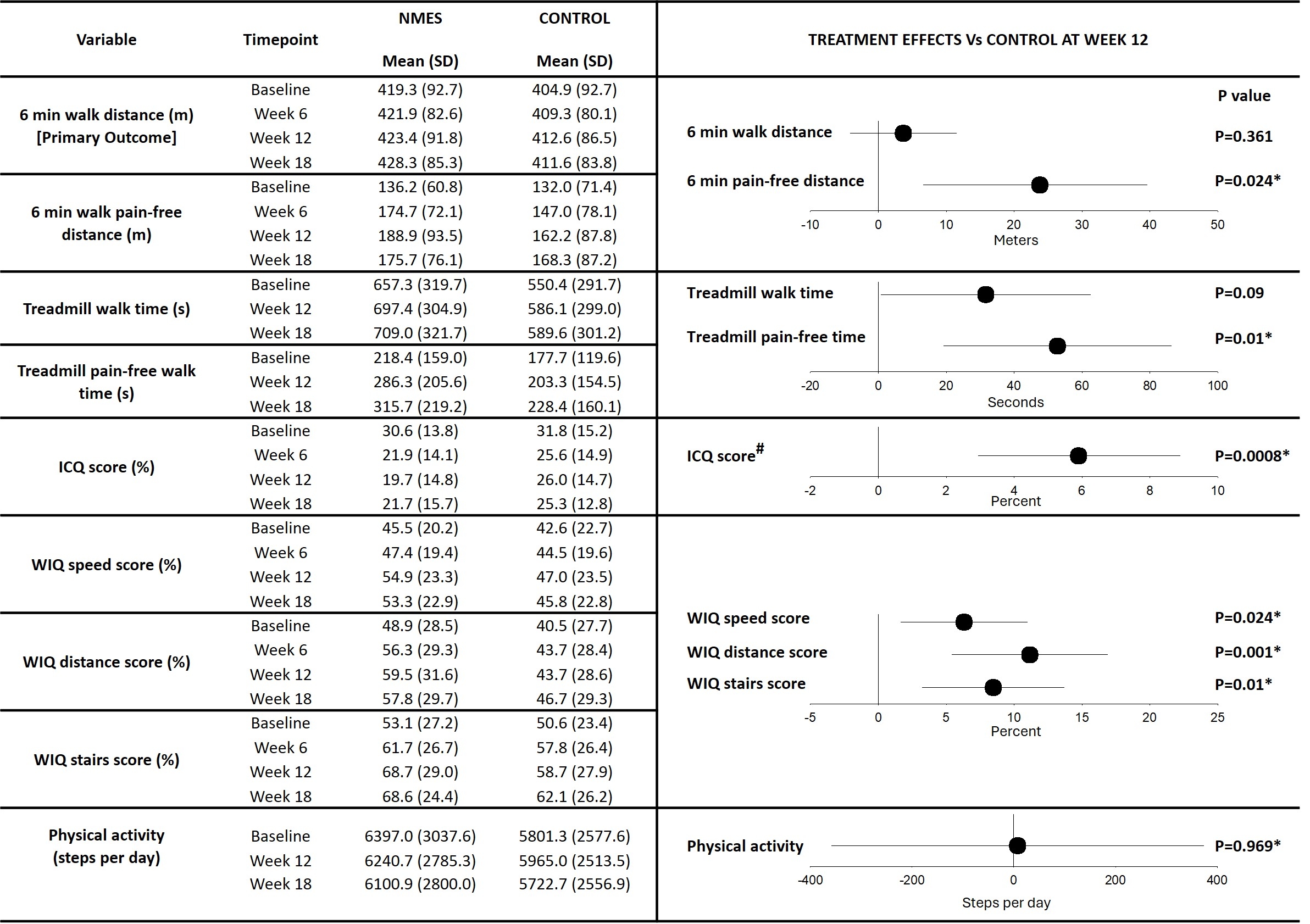
Outcomes: There was no difference in 6MWT distance between groups at week 12 (marginal mean difference (MMD): 3.68 m; 95% CI: -4.21 to 11.56; P = 0.361). At week 18, six weeks after the intervention ceased, 6MWT distance was greater in the NMES group compared with CON (MMD: 10.77 m; 95% CI: 2.82 to 18.72; P = 0.008). Pain-free 6MWT distance increased and was greater in the NMES group compared with CON at week 12 (MMD: 23.08 m; 95% CI: 6.55 to 39.61; P = 0.024).
Study Design and Methods: Single-centre, parallel-group, double-blinded, randomized placebo-controlled trial. Participants were randomized to a 12-week program of NMES or sham placebo-control (CON). This was followed by a 6-week off-intervention follow-up. Outcomes were assessed at baseline (week 0), midway (week 6) and at the end of the intervention period (week 12), and again at the end of the follow-up phase (week 18). Sample Size: 180 patients (n=90 per group) from public hospital, private hospital and community clinic recruitment sites. Population Studied: Adults (mean age: 72 ± 9 y; ABI: 0.69 ± 0.20) with a diagnosis of PAD who experience leg pain when walking (intermittent claudication). Interventions: Participants used a footplate-NMES device (Revitive Medic Coach) for 2x30 min periods each day to deliver stimulation sufficient to induce contraction of the leg muscles. Control participants used a sham device that delivers a low intensity stimulation that does not induce muscle contraction. Primary End Point: Maximum distance during the six-minute walk test (6MWT) at week 12. Secondary End Points: Pain-free distance during the 6MWT, maximum and pain-free walking time during a graded treadmill test, self-reported walking impairment questionnaire (WIQ), disease-specific QOL assessed using the intermittent claudication questionnaire (ICQ), and accelerometer derived physical activity levels. Power Calculation: At an alpha-level of 0.05 and a power of 0.80 the trial was powered to detect a 30 m change (effect size 0.33) in 6MWT distance in the NMES group compared with CON.
Outcomes: There was no difference in 6MWT distance between groups at week 12 (marginal mean difference (MMD): 3.68 m; 95% CI: -4.21 to 11.56; P = 0.361). At week 18, six weeks after the intervention ceased, 6MWT distance was greater in the NMES group compared with CON (MMD: 10.77 m; 95% CI: 2.82 to 18.72; P = 0.008). Pain-free 6MWT distance increased and was greater in the NMES group compared with CON at week 12 (MMD: 23.08 m; 95% CI: 6.55 to 39.61; P = 0.024).
More abstracts on this topic:
Adverse Pregnancy Outcomes Are Associated with Incident Peripheral Artery Disease, Results from the Women’s Health Initiative.
Jackson Elizabeth, Leblanc Erin, Haring Bernhard, Harrington Laura, Allison Matthew, Eaton Charles, Lamonte Michael, Hovey Kathleen, Andrews Chris, Wells Gretchen, Manson Joann, Levitan Emily, Spracklen Cassandra, Wild Robert
A Mechanistic Insight Into The Connection Between Metabolism And Differentiation In ACTA2 P. R179 Smooth Muscle CellsEsparza Pinelo Jose, Krenz Hannah, Chen Jessica, Kaw Anita, Milewicz Dianna, Kwartler Callie