Final ID: MP1224
Support duration as a prognostic indicator in temporary MCS: divergent outcomes based on the 14-day FDA approved duration
Abstract Body (Do not enter title and authors here): Background
Impella 5.5 is a temporary MCS device FDA-approved for use up to 14 days. Limited data suggest it may be safe beyond this. We aim assess outcomes based on the duration of Impella 5.5 support in heart failure cardiogenic shock (HFCS) patients at our institution.
Methods
We retrospectively reviewed all HFCS patients supported with Impella 5.5 at our institution between 2020-2024. Patients supported for >14 days were compared with those supported for ≤14 days. Categorical variables were analyzed with chi-square tests, continuous variables with t-tests or Mann-Whitney U. Logistic regression identified factors linked to clinical outcomes.
Results
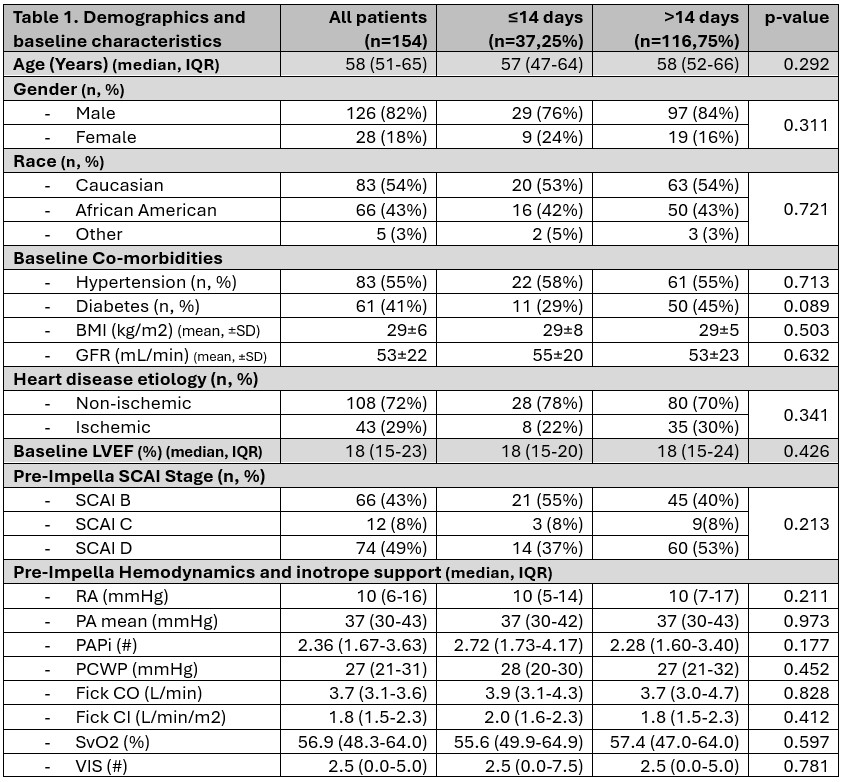
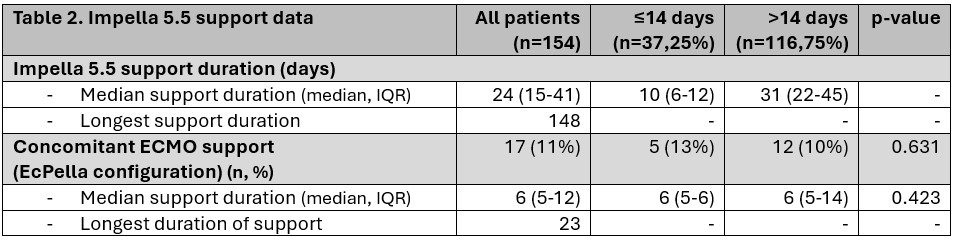
154 patients were included: 38 (25%) with support ≤14 days, 116 (75%) with >14 days. Median support duration was 10 (6–12) vs 31 (22–45) days, respectively. Median support duration was 10 (6-12) days for the ≤14 cohort and 31 days (22-45) days for the >14 cohort. Baseline characteristics, comorbidities, LVEF 18% (15-23) and hemodynamics show no statistical differences.
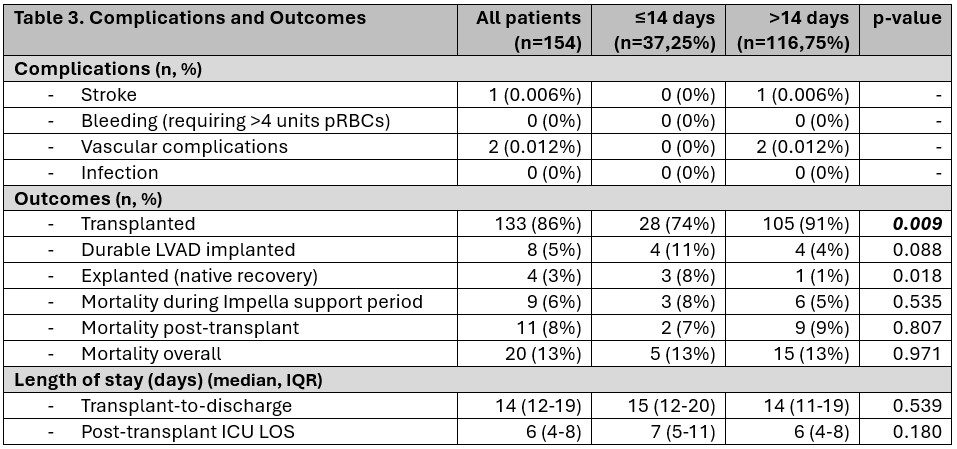
Patients supported for >14 days were more likely to undergo heart transplant compared to those supported ≤14 days (91% vs 74%, p=0.009). In contrast, myocardial recovery was more likely in the ≤14 day cohort (8% vs 1%, p=0.018). One patient suffered stroke post-transplant in the ≤14 day group and two had brachial plexus injuries in the >14 days group. Mortality during the impella-supported period was slightly higher in the ≤14 day group (8% vs 5%, p=0.535) but comparable overall, 13% in both groups, p=0.971.
Support >14 days was associated with transplant outcome (OR 3.41, 95% CI 1.32–8.84, p=0.012), and remained significant after adjusting for age, gender, GFR, and EF (OR 4.05, 95% CI 1.31–12.54, p=0.015). Support duration <14 days was associated with recovery or LVAD outcomes (OR 5.01, 95% CI 1.49–16.89, p=0.009), and remained significant after adjustment (OR 5.43, 95% CI 1.11–26.45, p=0.036). No association was observed between duration of support and LVAD/mortality outcomes or with EcPella use.
Conclusion
Shorter Impella support duration was independently associated with early myocardial recovery or LVAD candidacy, likely reflecting rapid clinical decision-making. Prolonged Impella support was associated with increased likelihood of transitioning to heart transplantation, likely reflecting clinical stabilization over time. Our multidisciplinary, single-center data suggests Impella 5.5 can be used beyond FDA-approved 14 days in HFCS patients.
Impella 5.5 is a temporary MCS device FDA-approved for use up to 14 days. Limited data suggest it may be safe beyond this. We aim assess outcomes based on the duration of Impella 5.5 support in heart failure cardiogenic shock (HFCS) patients at our institution.
Methods
We retrospectively reviewed all HFCS patients supported with Impella 5.5 at our institution between 2020-2024. Patients supported for >14 days were compared with those supported for ≤14 days. Categorical variables were analyzed with chi-square tests, continuous variables with t-tests or Mann-Whitney U. Logistic regression identified factors linked to clinical outcomes.
Results
154 patients were included: 38 (25%) with support ≤14 days, 116 (75%) with >14 days. Median support duration was 10 (6–12) vs 31 (22–45) days, respectively. Median support duration was 10 (6-12) days for the ≤14 cohort and 31 days (22-45) days for the >14 cohort. Baseline characteristics, comorbidities, LVEF 18% (15-23) and hemodynamics show no statistical differences.
Patients supported for >14 days were more likely to undergo heart transplant compared to those supported ≤14 days (91% vs 74%, p=0.009). In contrast, myocardial recovery was more likely in the ≤14 day cohort (8% vs 1%, p=0.018). One patient suffered stroke post-transplant in the ≤14 day group and two had brachial plexus injuries in the >14 days group. Mortality during the impella-supported period was slightly higher in the ≤14 day group (8% vs 5%, p=0.535) but comparable overall, 13% in both groups, p=0.971.
Support >14 days was associated with transplant outcome (OR 3.41, 95% CI 1.32–8.84, p=0.012), and remained significant after adjusting for age, gender, GFR, and EF (OR 4.05, 95% CI 1.31–12.54, p=0.015). Support duration <14 days was associated with recovery or LVAD outcomes (OR 5.01, 95% CI 1.49–16.89, p=0.009), and remained significant after adjustment (OR 5.43, 95% CI 1.11–26.45, p=0.036). No association was observed between duration of support and LVAD/mortality outcomes or with EcPella use.
Conclusion
Shorter Impella support duration was independently associated with early myocardial recovery or LVAD candidacy, likely reflecting rapid clinical decision-making. Prolonged Impella support was associated with increased likelihood of transitioning to heart transplantation, likely reflecting clinical stabilization over time. Our multidisciplinary, single-center data suggests Impella 5.5 can be used beyond FDA-approved 14 days in HFCS patients.
More abstracts on this topic:
6-Nitrodopamine potentiates the positive chronotopic and inotropic effect induced by noradrenaline in the rat isolated heart
Lima Antonio, Sobanski Joao Fernando, Antunes Edson, De Nucci Gilberto
Assessing the Clinical Impact of Cardiac Intensivists in Cardiac Intensive Care Units.: Results from the RESCUE registryBae Dae-hwan, Bae Jang-whan, Lee Junyoung, Sun Jonghee, Lee Sang Yeub, Yang Jeong Hoon, Gwon Hyeon-cheol