Final ID: Mo4022
Characterizing Shared Genetic Architectures in Brain and Cardiovascular Diseases via Clustering of Survival GWAS Summary Statistics
Abstract Body (Do not enter title and authors here):
Background:
Clustering neurological, psychiatric, and cardiovascular diseases based on GWAS summary statistics offers opportunities to uncover their shared genetic architecture across the brain–heart axis. Survival GWAS improves biological and clinical resolution over traditional binary trait GWAS by modeling disease age-of-onset as time-to-event (TTE) traits.
Research Question:
Can unsupervised clustering of survival GWAS summary statistics uncover biologically coherent disease groups? If so, can we identify cluster-specific hallmark genes that reflect shared genetic architecture and inform downstream analyses such as pathway discovery and therapeutic repurposing?
Methods:
We selected 64 brain and cardiovascular diseases using distinct one-decimal sub-phecodes. For each TTE trait approximated by age at diagnosis, we extracted Z-scores for 1.28 million HapMap3 SNPs using Genetic Analysis of Time-to-Event phenotypes (GATE), which applied a frailty-adjusted Cox to 408,582 White British participants in the UK Biobank. Trait clustering was performed using HDBSCAN (a hierarchical density-based clustering method) on the first 15 principal components of the Z-scores to emphasize low-rank genetic structure while reducing noise. Within each resulting cluster, we conducted fixed-effect meta-analysis across traits to generate cluster-level GWAS summary statistics, which were then analyzed using MAGMA to assess gene-level associations.
Results:
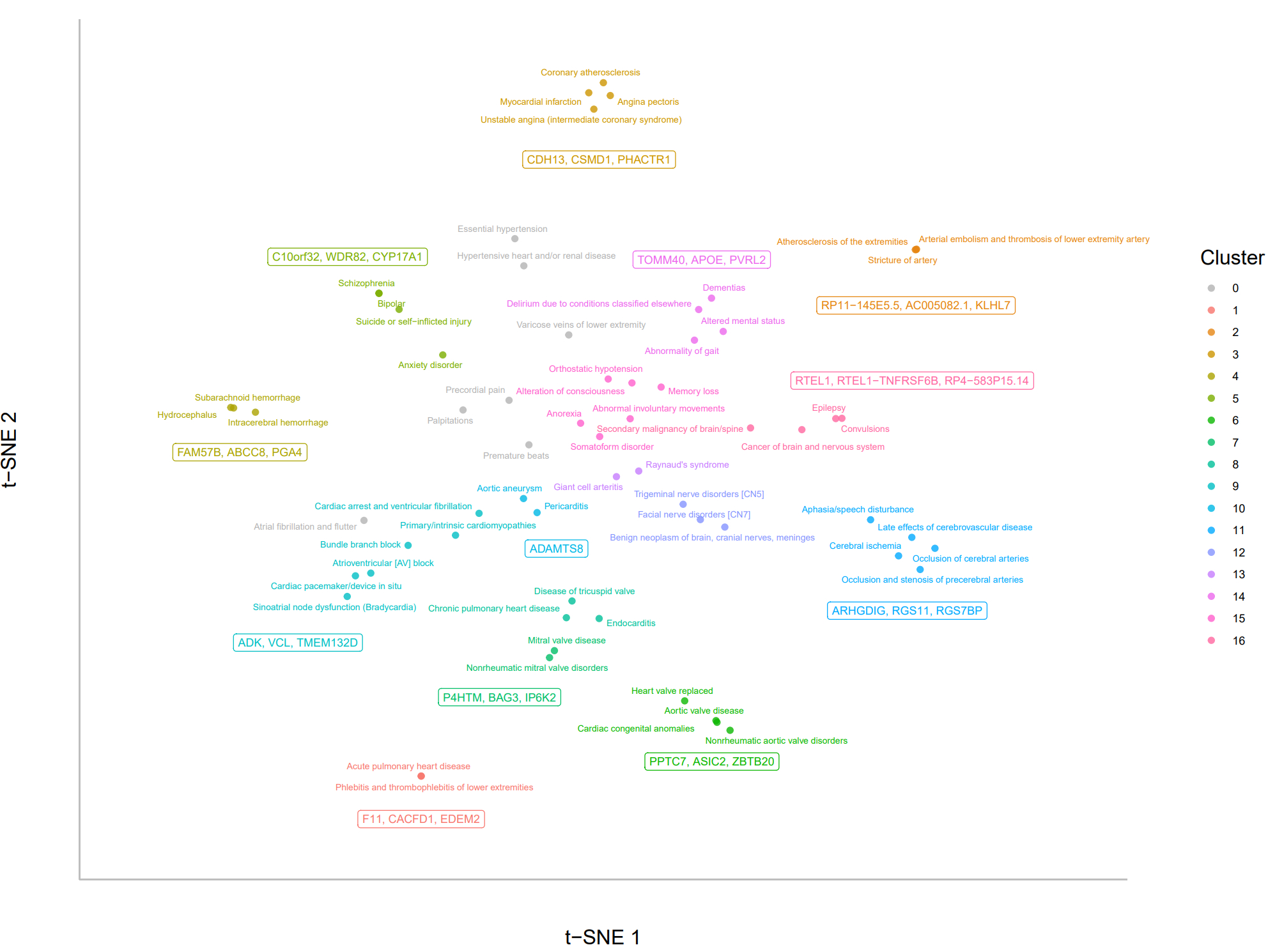
We identified 16 disease clusters and reported top three significant genes (FDR < 0.05) for each cluster, visualized using a t-SNE plot (Figure). Several clusters yielded biologically coherent disease grouping and gene signals. For example, the coronary artery disease cluster highlighted CDH13, CSMD1, and PHACTR1; the dementia cluster revealed TOMM40, APOE, and PVRL2; and the conduction disorder cluster identified ADK, VCL and TMEM132D. These genes participate in vascular, neurodegenerative, and electrophysiological processes aligned with their respective disease clusters. In contrast, clusters composed of symptom-based or heterogeneous traits (e.g., palpitations and hypertension) showed no significant gene hits.
Conclusions:
Clustering TTE traits based on disease age-of-onset-aware GWAS summary statistics can reveal convergent biology across the brain–heart axis. The identification of well-established loci supports this approach as a framework for pathway discovery and therapeutic repurposing in genetically overlapping conditions.
Background:
Clustering neurological, psychiatric, and cardiovascular diseases based on GWAS summary statistics offers opportunities to uncover their shared genetic architecture across the brain–heart axis. Survival GWAS improves biological and clinical resolution over traditional binary trait GWAS by modeling disease age-of-onset as time-to-event (TTE) traits.
Research Question:
Can unsupervised clustering of survival GWAS summary statistics uncover biologically coherent disease groups? If so, can we identify cluster-specific hallmark genes that reflect shared genetic architecture and inform downstream analyses such as pathway discovery and therapeutic repurposing?
Methods:
We selected 64 brain and cardiovascular diseases using distinct one-decimal sub-phecodes. For each TTE trait approximated by age at diagnosis, we extracted Z-scores for 1.28 million HapMap3 SNPs using Genetic Analysis of Time-to-Event phenotypes (GATE), which applied a frailty-adjusted Cox to 408,582 White British participants in the UK Biobank. Trait clustering was performed using HDBSCAN (a hierarchical density-based clustering method) on the first 15 principal components of the Z-scores to emphasize low-rank genetic structure while reducing noise. Within each resulting cluster, we conducted fixed-effect meta-analysis across traits to generate cluster-level GWAS summary statistics, which were then analyzed using MAGMA to assess gene-level associations.
Results:
We identified 16 disease clusters and reported top three significant genes (FDR < 0.05) for each cluster, visualized using a t-SNE plot (Figure). Several clusters yielded biologically coherent disease grouping and gene signals. For example, the coronary artery disease cluster highlighted CDH13, CSMD1, and PHACTR1; the dementia cluster revealed TOMM40, APOE, and PVRL2; and the conduction disorder cluster identified ADK, VCL and TMEM132D. These genes participate in vascular, neurodegenerative, and electrophysiological processes aligned with their respective disease clusters. In contrast, clusters composed of symptom-based or heterogeneous traits (e.g., palpitations and hypertension) showed no significant gene hits.
Conclusions:
Clustering TTE traits based on disease age-of-onset-aware GWAS summary statistics can reveal convergent biology across the brain–heart axis. The identification of well-established loci supports this approach as a framework for pathway discovery and therapeutic repurposing in genetically overlapping conditions.
More abstracts on this topic:
Alterations in Heart Rate Variability After Ischemic Stroke in Rats: Heart-Brain Connectivity
Alavi Rashid, Dai Wangde, Li Jiajun, Carreno Juan, Pahlevan Niema, Arakaki Xianghong, Gharib Morteza, Kloner Robert
A Multi-Tier, Natural-Language Processing Framework to Automate Labeling of Acute Cerebrovascular Events From Radiology Reports and Diagnosis CodesErekat Asala, Stein Laura, Delman Bradley, Karp Adam, Kupersmith Mark, Kummer Benjamin