Final ID: MP2404
Longitudinal Evaluation of Anti-Arrhythmic Drug Use to Predict Hospitalization or Death in Patients with Ventricular Tachycardia
Abstract Body (Do not enter title and authors here): Introduction: Anti-arrhythmic drugs in patients with VT are chosen based on comorbidity profile. However, the relationship between comorbidity profile, selection of drugs and hard clinical endpoints in patients with VT is poorly understood. We set out to survey this in a large registry of over 14,000 patients.
Hypothesis: Statistical classification will identify patients with VT who are more likely to respond to individual AADs, as measured by hospitalization and mortality endpoints.
Methods: We retrospectively analyzed a registry of 14,964 patients with ventricular tachycardia at our center (mean age 65.7 ± 16.5 years; 37.2% female) between 1988-2022. Baseline demographics and comorbidities were assessed at VT diagnosis date. From then, use of AADs (beta-blockers, sotalol, amiodarone, dofetilide, mexiletine) in the three years following were assessed on a quarterly basis. Cox proportional hazards models for both cardiovascular (CV) hospitalization and all-cause mortality were used to evaluate predictors. Individual AADs were modeled as time-varying covariates, and patients were censored if death or loss to follow-up occurred before the end of three-year follow-up.
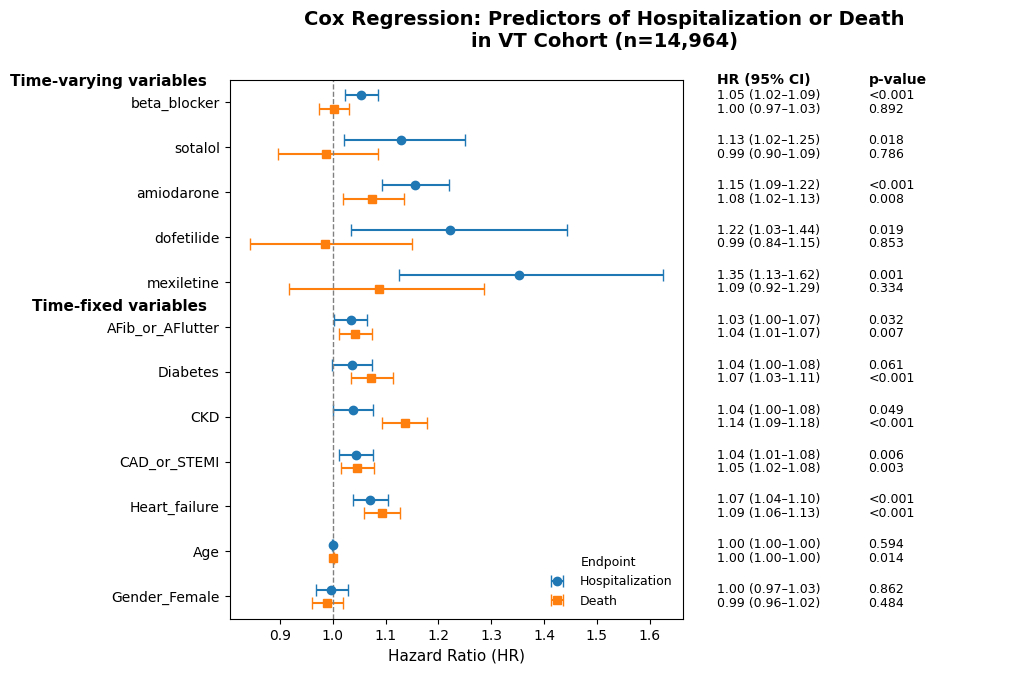
Results: Initially, a univariate analysis of 65 comorbidities was performed for association with CV hospitalization or mortality. Age, gender, and variables with a p<0.10 for either endpoint were then included in multivariate analysis. For CV hospitalization, only comorbidities of heart failure and CAD were significant predictors - notably, age, gender, CKD, diabetes, and atrial fibrillation/flutter were not. For mortality, of AADs only amiodarone predicted death while multiple comorbidities were significant predictors.
Patients on amiodarone had mean age 67.4±14.1 years and a higher proportion of co-morbidities (mean number: 3.7±2.1 versus 2.97±2.0, p<0.001) than those on other anti-arrhythmic medications of sotalol, dofetilide, beta-blockers, and mexiletine, and therefore had a higher association with mortality. As expected, all anti-arrhythmics were associated with hospitalization, reflecting their inpatient initiation or use in patients who were previously hospitalized.
Conclusions: Linking AAD use with clinical outcomes is an important first step in identifying which patient profiles respond to which individual AADs and developing tailored VT treatment approaches. Here, we demonstrate profiles of VT patients at higher risk for hospitalization or death based on individual AADs.
Hypothesis: Statistical classification will identify patients with VT who are more likely to respond to individual AADs, as measured by hospitalization and mortality endpoints.
Methods: We retrospectively analyzed a registry of 14,964 patients with ventricular tachycardia at our center (mean age 65.7 ± 16.5 years; 37.2% female) between 1988-2022. Baseline demographics and comorbidities were assessed at VT diagnosis date. From then, use of AADs (beta-blockers, sotalol, amiodarone, dofetilide, mexiletine) in the three years following were assessed on a quarterly basis. Cox proportional hazards models for both cardiovascular (CV) hospitalization and all-cause mortality were used to evaluate predictors. Individual AADs were modeled as time-varying covariates, and patients were censored if death or loss to follow-up occurred before the end of three-year follow-up.
Results: Initially, a univariate analysis of 65 comorbidities was performed for association with CV hospitalization or mortality. Age, gender, and variables with a p<0.10 for either endpoint were then included in multivariate analysis. For CV hospitalization, only comorbidities of heart failure and CAD were significant predictors - notably, age, gender, CKD, diabetes, and atrial fibrillation/flutter were not. For mortality, of AADs only amiodarone predicted death while multiple comorbidities were significant predictors.
Patients on amiodarone had mean age 67.4±14.1 years and a higher proportion of co-morbidities (mean number: 3.7±2.1 versus 2.97±2.0, p<0.001) than those on other anti-arrhythmic medications of sotalol, dofetilide, beta-blockers, and mexiletine, and therefore had a higher association with mortality. As expected, all anti-arrhythmics were associated with hospitalization, reflecting their inpatient initiation or use in patients who were previously hospitalized.
Conclusions: Linking AAD use with clinical outcomes is an important first step in identifying which patient profiles respond to which individual AADs and developing tailored VT treatment approaches. Here, we demonstrate profiles of VT patients at higher risk for hospitalization or death based on individual AADs.
More abstracts on this topic:
Age-stratified Monogenic and Polygenic Contributions for Atrial Fibrillation in the All of Us Research Program
Chen Zhanlin, Gordon Adam, Webster Gregory
Accuracy of Rule-based Natural Language Processing Models for Identification of Pulmonary EmbolismRashedi Sina, Jimenez David, Monreal Manuel, Secemsky Eric, Klok Erik, Hunsaker Andetta, Aghayev Ayaz, Muriel Alfonso, Hussain Mohamad, Appah-sampong Abena, Aneja Sanjay, Krishnathasan Darsiya, Mojibian Hamid, Goldhaber Samuel, Wang Liqin, Zhou Li, Krumholz Harlan, Piazza Gregory, Bikdeli Behnood, Khairani Candrika, Bejjani Antoine, Lo Ying-chih, Zarghami Mehrdad, Mahajan Shiwani, Caraballo Cesar, Jimenez Ceja Jose Victor