Final ID: MP1620
Impact of Donor and Recipient Risk Matching on Survival After Pediatric Heart Transplantation
Abstract Body (Do not enter title and authors here): Background
Pediatric heart transplantation (HT) is limited by donor numbers and graft quality, although recipient clinical acuity may have the greatest impact on post-HT survival. Increasingly, more marginal donors are being used with reasonable outcomes, but often these are implanted into the sickest recipients. If acceptance of higher risk donors could provide excellent post-HT outcomes for low-risk recipients, then this could improve utilization of marginal donors while also increasing availability of lower risk donors for high-risk recipients.
Methods
A retrospective cohort analysis of the Pediatric Heart Transplant Society (PHTS) database was performed for all pediatric (age <18 years) patients (n=5920) undergoing primary HT from 1/1/2010−6/30/2024. Separate donor and recipient risk scores were developed using multivariable multiphase parametric hazard modeling. Patients were stratified into low-, medium-, or high-risk categories for each donor-recipient pair, according to tertiles of the predicted 1-year survival estimates from the risk models.
Results
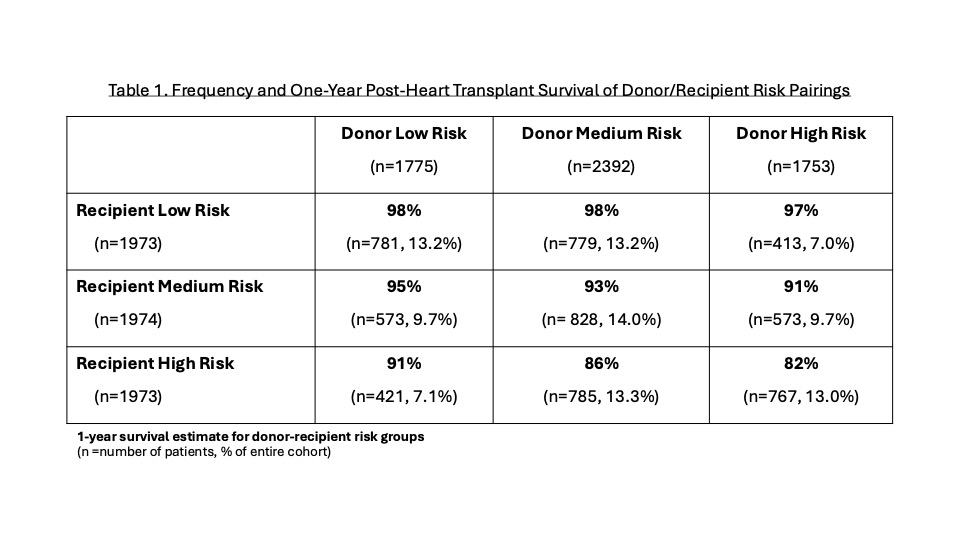
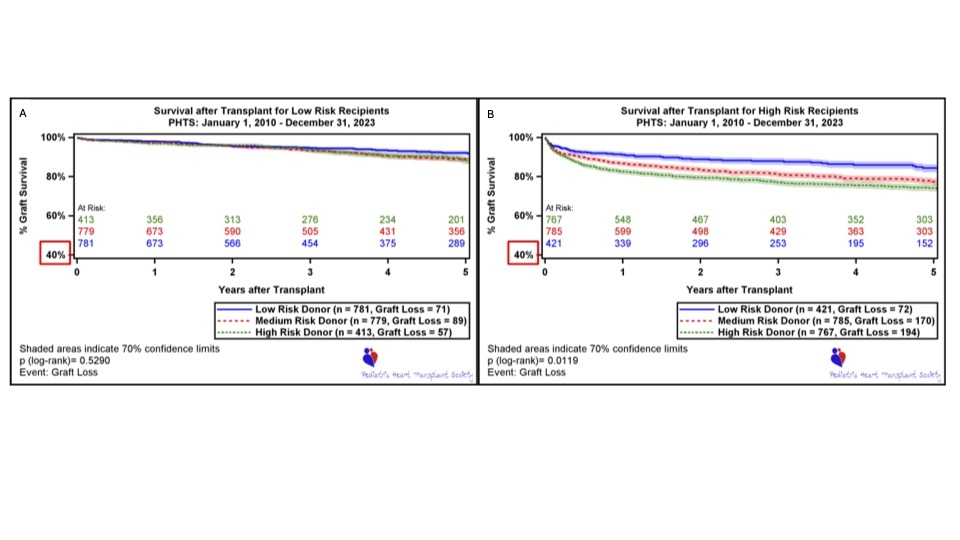
Overall, 1-year post-HT survival was 92%. Donor risk factors included age <3 vs 3-17 years (HR 1.74), oversized height vs well-matched (HR 1.97) and head trauma as cause of death (HR 0.69) (p<0.05 for all). Recipient risk factors included congenital heart disease (HR 3.91), age (HR 0.98), panel reactive antibodies >10% (HR 1.46), waitlist interval (HR 1.16), renal dysfunction (HR 1.87), induction therapy (HR 0.69), ventilator (HR 1.46), extracorporeal membrane oxygenation (HR 3.65), and any ventricular assist device (HR 1.66) at time of HT (p<0.05 for all). Low-risk recipients tended to be matched with low- or medium-risk donors, while high-risk recipients tended to be matched with medium- or high-risk donors (Table 1). Low-risk recipients had similar 1-year survival regardless of donor risk, while high-risk recipients had worst 1-year survival with medium- or high-risk donors (Figure 1).
Conclusion
Recipient risk profile has a greater impact on 1-year graft survival compared to donor risk. For low-risk recipients, donor risk profile has less impact on survival. By increasing the use of marginal donor organs for low-risk recipients and thereby increasing availability of low-risk organs for the sickest recipients, it may be possible to decrease waitlist mortality and improve post-HT outcomes for the entire cohort.
Pediatric heart transplantation (HT) is limited by donor numbers and graft quality, although recipient clinical acuity may have the greatest impact on post-HT survival. Increasingly, more marginal donors are being used with reasonable outcomes, but often these are implanted into the sickest recipients. If acceptance of higher risk donors could provide excellent post-HT outcomes for low-risk recipients, then this could improve utilization of marginal donors while also increasing availability of lower risk donors for high-risk recipients.
Methods
A retrospective cohort analysis of the Pediatric Heart Transplant Society (PHTS) database was performed for all pediatric (age <18 years) patients (n=5920) undergoing primary HT from 1/1/2010−6/30/2024. Separate donor and recipient risk scores were developed using multivariable multiphase parametric hazard modeling. Patients were stratified into low-, medium-, or high-risk categories for each donor-recipient pair, according to tertiles of the predicted 1-year survival estimates from the risk models.
Results
Overall, 1-year post-HT survival was 92%. Donor risk factors included age <3 vs 3-17 years (HR 1.74), oversized height vs well-matched (HR 1.97) and head trauma as cause of death (HR 0.69) (p<0.05 for all). Recipient risk factors included congenital heart disease (HR 3.91), age (HR 0.98), panel reactive antibodies >10% (HR 1.46), waitlist interval (HR 1.16), renal dysfunction (HR 1.87), induction therapy (HR 0.69), ventilator (HR 1.46), extracorporeal membrane oxygenation (HR 3.65), and any ventricular assist device (HR 1.66) at time of HT (p<0.05 for all). Low-risk recipients tended to be matched with low- or medium-risk donors, while high-risk recipients tended to be matched with medium- or high-risk donors (Table 1). Low-risk recipients had similar 1-year survival regardless of donor risk, while high-risk recipients had worst 1-year survival with medium- or high-risk donors (Figure 1).
Conclusion
Recipient risk profile has a greater impact on 1-year graft survival compared to donor risk. For low-risk recipients, donor risk profile has less impact on survival. By increasing the use of marginal donor organs for low-risk recipients and thereby increasing availability of low-risk organs for the sickest recipients, it may be possible to decrease waitlist mortality and improve post-HT outcomes for the entire cohort.
More abstracts on this topic:
Application of Artificial Intelligence (AI) for Predictive Modelling and Imaging in Cardiac Transplantation - A Systematic Review and Meta-Analysis
Iyer Vardhini Ganesh, Chandra Mohan Trisha, Gupta Aryan, Gupta Era, Prasad Kushal, Kalra Shekhar, Chandramouli Bellur Vinay, Prasad Ananya, Oudit Omar, Magaji Rishikesh R
Afterschool Rx: A Feasibility Study of a Community-Based Prescription for Reducing Cardiovascular RiskVon Klinggraeff Lauren, Beets Michael, Lane Abbi, Garimella Sudha, Armstrong Bridget, Parker Hannah, Patel Isha, Smith Michal, Coughlin Steven, Davis Catherine, Estabrooks Paul, Harris Ryan, Vernon Marlo