Final ID: MP2444
Long-Term Survival in Pediatric Heart Transplantation: A PHTS-SRTR Linkage Study
Abstract Body (Do not enter title and authors here): BACKGROUND: There is a growing population of adult survivors after pediatric heart transplantation (HTx). Little is known about long-term outcomes, particularly after transition to adult care. We aimed to: (1) characterize the survival of pediatric HTx recipients and (2) identify risk factors associated with mortality in long-term survivors.
METHODS: Data from the Pediatric Heart Transplant Society (PHTS) registry were linked using direct identifiers to the Scientific Registry of Transplant Recipients (SRTR) to identify patients (aged ≤18 years) who underwent first HTx between 1993 and 2020. Patients with ≥3 years of PHTS follow-up were included in the risk factor analysis. The primary outcome was patient death in the SRTR, which includes verified deaths from the Social Security Administration Death Master File. Kaplan-Meier analysis, competing risk analysis, and Cox proportional hazard modeling were used to assess survival and identify pre- and early post-HTx risk factors for mortality for 3-year conditional survivors.
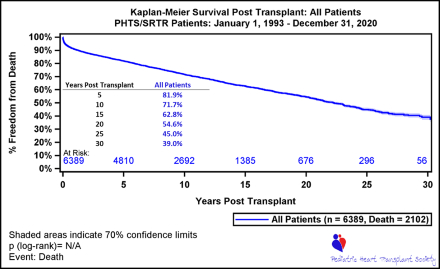
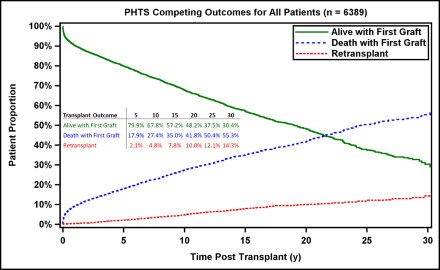
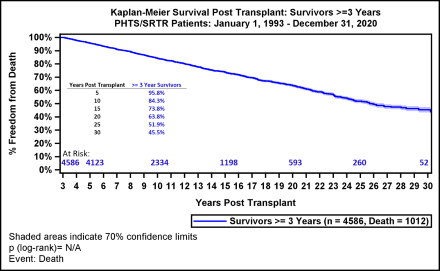
RESULTS: A 94% linkage rate was achieved between the two databases (n=6389). The mean age at HTx was 6.6 years, and 3227 (51%) had congenital heart disease. Median survival was 22 years, and the retransplant rate was 14% at 30 years. For 3-year conditional survivors, the 30-year survival was 46%. In this group, both infants and single ventricle patients who underwent primary HTx had a 30-year survival of 58%. Common causes of death were non-rejection cardiovascular in 367 (36%), rejection in 181 (18%) and malignancy or PTLD in 62 (6%). Independent risk factors for mortality included older age at HTx, black race, donor sex mismatch, mechanical ventilation at HTx, higher steroid exposure, number of rejection episodes and recent diagnosis of cardiac allograft vasculopathy (p<0.05 for all). Patients transplanted between 11-18 years and with 3+ rejection episodes in the first 3 years had the highest hazard of mortality (HR 2.2, 95% CI 1.8-2.7 and HR 2.2, 95% CI 1.8-2.8).
CONCLUSIONS: We established a novel and robust data linkage, enabling the longest-term survival analysis of pediatric HTx recipients to date. Pediatric HTx is an effective therapy that allows half of patients to survive ≥22 years. Age at HTx and early rejection are more influential on long-term survival than underlying diagnosis or stage of palliation. Increasing retransplant rates and preventing early rejection are strategies which may improve long-term pediatric HTx survival.
METHODS: Data from the Pediatric Heart Transplant Society (PHTS) registry were linked using direct identifiers to the Scientific Registry of Transplant Recipients (SRTR) to identify patients (aged ≤18 years) who underwent first HTx between 1993 and 2020. Patients with ≥3 years of PHTS follow-up were included in the risk factor analysis. The primary outcome was patient death in the SRTR, which includes verified deaths from the Social Security Administration Death Master File. Kaplan-Meier analysis, competing risk analysis, and Cox proportional hazard modeling were used to assess survival and identify pre- and early post-HTx risk factors for mortality for 3-year conditional survivors.
RESULTS: A 94% linkage rate was achieved between the two databases (n=6389). The mean age at HTx was 6.6 years, and 3227 (51%) had congenital heart disease. Median survival was 22 years, and the retransplant rate was 14% at 30 years. For 3-year conditional survivors, the 30-year survival was 46%. In this group, both infants and single ventricle patients who underwent primary HTx had a 30-year survival of 58%. Common causes of death were non-rejection cardiovascular in 367 (36%), rejection in 181 (18%) and malignancy or PTLD in 62 (6%). Independent risk factors for mortality included older age at HTx, black race, donor sex mismatch, mechanical ventilation at HTx, higher steroid exposure, number of rejection episodes and recent diagnosis of cardiac allograft vasculopathy (p<0.05 for all). Patients transplanted between 11-18 years and with 3+ rejection episodes in the first 3 years had the highest hazard of mortality (HR 2.2, 95% CI 1.8-2.7 and HR 2.2, 95% CI 1.8-2.8).
CONCLUSIONS: We established a novel and robust data linkage, enabling the longest-term survival analysis of pediatric HTx recipients to date. Pediatric HTx is an effective therapy that allows half of patients to survive ≥22 years. Age at HTx and early rejection are more influential on long-term survival than underlying diagnosis or stage of palliation. Increasing retransplant rates and preventing early rejection are strategies which may improve long-term pediatric HTx survival.
More abstracts on this topic:
30-day and one-year outcomes of patients with severe aortic stenosis after TAVI using Myval : A Meta-analysis
Hasabo Elfatih A., Sultan Sherif, Soliman Osama, A. Aboali Amira, Hemmeda Lina, Salah Alaa, Alrawa Salma S., Elgadi Ammar, Abdalmotalib Malaz, Yasir H Eissa Abdullatif, Mahmmoud Fadelallah Eljack Mohammed
A Non-Contacting Blood Flow Sensor for Assessing Pediatric Vascular Graft PatencyChen Ruitong, Hudson Trevor, Wang Xuechun, Liang Jingjing, Nowlen Alanna, Nigam Vishal, Meng Ellis