Final ID: MP1629
Associations of baseline and longitudinal changes in MASLD with risk of heart failure events in type 2 diabetes and overweight or obesity: the Look Action for Health in Diabetes (Look AHEAD) Trial Liver Ancillary Study
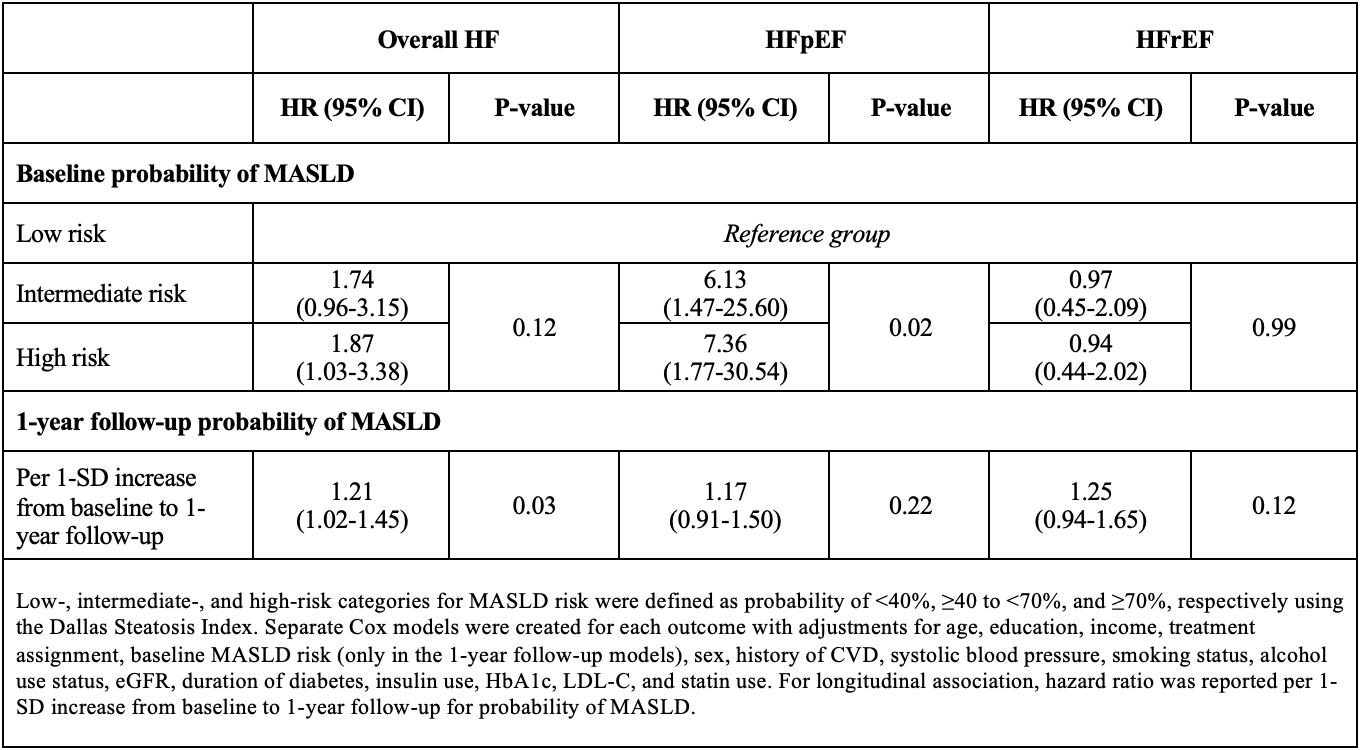
Methods: We included participants with T2D and overweight/obesity from the Look AHEAD (Action for Health in Diabetes) trial who had available AST, ALT, and GGT data. The probability of MASLD was estimated using the Dallas Steatosis Index and classified as low, intermediate, or high based on probabilities <40%, ≥40 to <70%, and ≥70%, respectively. Adjusted Cox models were created to evaluate the associations of baseline and 1-year longitudinal changes in probability of MASLD with risk of overall HF, HF with preserved and reduced ejection fraction (HFpEF and HFrEF, respectively).
Results: The present study included 3,938 participants (mean age 59 years, 59% female, 67% White race). During follow-up, 211 HF events occurred (107 HFpEF, 84 HFrEF). Compared with a low probability of MASLD at baseline, high probability of MASLD at baseline was associated with overall HF risk (Table). In HF subtype analyses, higher probability of MASLD was significantly associated with higher risk of HFpEF but not HFrEF. In longitudinal analysis, 1-year increase in the probability of MASLD was significantly associated with higher risk of overall HF with a similar, non-significant pattern of association observed for both HF subtypes.
Conclusions: Among adults with T2D and overweight or obesity in the Look AHEAD trial, higher probability of MASLD at baseline and increase in MASLD probability over 1-year follow-up were associated with higher risk of overall HF. Baseline MASLD probability was significantly associated with risk of HFpEF but not HFrEF. These findings underscore the prognostic implications of MASLD and its importance in HF risk assessment in T2D.
- Chunawala, Zainali ( University of Texas Southwestern , Dallas , Texas , United States )
- Butler, Javed ( Baylor Scott and White , Dallas , Texas , United States )
- Ballantyne, Christie ( BAYLOR COLLEGE MEDICINE , Houston , Texas , United States )
- Bertoni, Alain ( WAKE FOREST UNIV SCHOOL MED , Pfafftown , North Carolina , United States )
- Espeland, Mark ( Wake Forest School of Medicine , Winston-Salem , North Carolina , United States )
- De Lemos, James ( UT SOUTHWESTERN MEDICAL CTR , Dallas , Texas , United States )
- Pandey, Ambarish ( UT Southwestern Medical Center , Dallas , Texas , United States )
- Patel, Kershaw ( Houston Methodist Hospital , Houston , Texas , United States )
- Clark, Jeanne ( Rutgers Robert Wood Johnson Med Sch , New Brunswick , New Jersey , United States )
- Vanwagner, Lisa ( University of Texas Southwestern , Dallas , Texas , United States )
- Browning, Jeffrey ( University of Texas Southwestern , Dallas , Texas , United States )
- Garcia, Katelyn ( Wake Forest Baptist Health , Winston-Salem , North Carolina , United States )
- Zannad, Faiez ( CVCT and Universite de Lorraine , Paris , France )
- Maruthur, Nisa ( John Hopkins , Dallas , Maryland , United States )
- Sanyal, Arun ( Virginia Commonwealth University , Richmond , Virginia , United States )
Meeting Info:
Session Info:
Drivers of Heart Failure: Environmental and Behavioral Factors
Sunday, 11/09/2025 , 09:15AM - 10:30AM
Moderated Digital Poster Session
More abstracts on this topic:
De Jong Vivian, Grobbee Diederick, Saidi Alina, Castro Cabezas Manuel, Nissen Steven, Sasiela William, Li Na, Bloedon Leanne, Lincoff Abraham, Nicholls Stephen
A community-engaged approach to culturally tailoring a dietary intervention to improve cardiometabolic health among Black adults with obesity in Los Angeles CountyAdeyemo Mopelola, Thorpe Roland
More abstracts from these authors:
Chunawala Zainali, Butler Javed, Ballantyne Christie, Bertoni Alain, Espeland Mark, De Lemos James, Pandey Ambarish, Patel Kershaw, Clark Jeanne, Vanwagner Lisa, Browning Jeffrey, Garcia Katelyn, Zannad Faiez, Maruthur Nisa, Sanyal Arun
Intensive lifestyle intervention, cardiac biomarkers and atherosclerotic cardiovascular disease in type 2 diabetes and overweight or obesity – a post-hoc analysis of the Look Action for Health in Diabetes (AHEAD) trialPatel Kershaw, Bertoni Alain, Espeland Mark, Pandey Ambarish, Chunawala Zainali, Segar Matthew, Garcia Katelyn, Ndumele Chiadi, Wang Thomas, Januzzi James, Butler Javed, Lam Carolyn