Final ID: 4367950
Intensive lifestyle intervention, MASLD, and cardiovascular outcomes in type 2 diabetes and overweight or obesity: the Look Action for Health in Diabetes (Look AHEAD) trial Liver Ancillary Study
Methods: Look AHEAD (Action for Health in Diabetes) trial participants with T2D and overweight or obesity were included. AST, ALT, and GGT were measured at baseline and 1-year follow-up. The probability of MASLD was calculated using the Dallas Steatosis Index (DSI) and the Framingham Steatosis Index (FSI) which were compared with proton magnetic resonance spectroscopy (1H MRS) in a subset of participants with 244 studies. MASLD was defined as hepatic steatosis ≥5.5%. The effect of the ILI versus diabetes support and education (DSE) on measures of MASLD were assessed using least-square means. Adjusted Cox models were created to evaluate the associations of baseline and 1-year longitudinal changes in probability of MASLD with risk of ASCVD (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for angina).
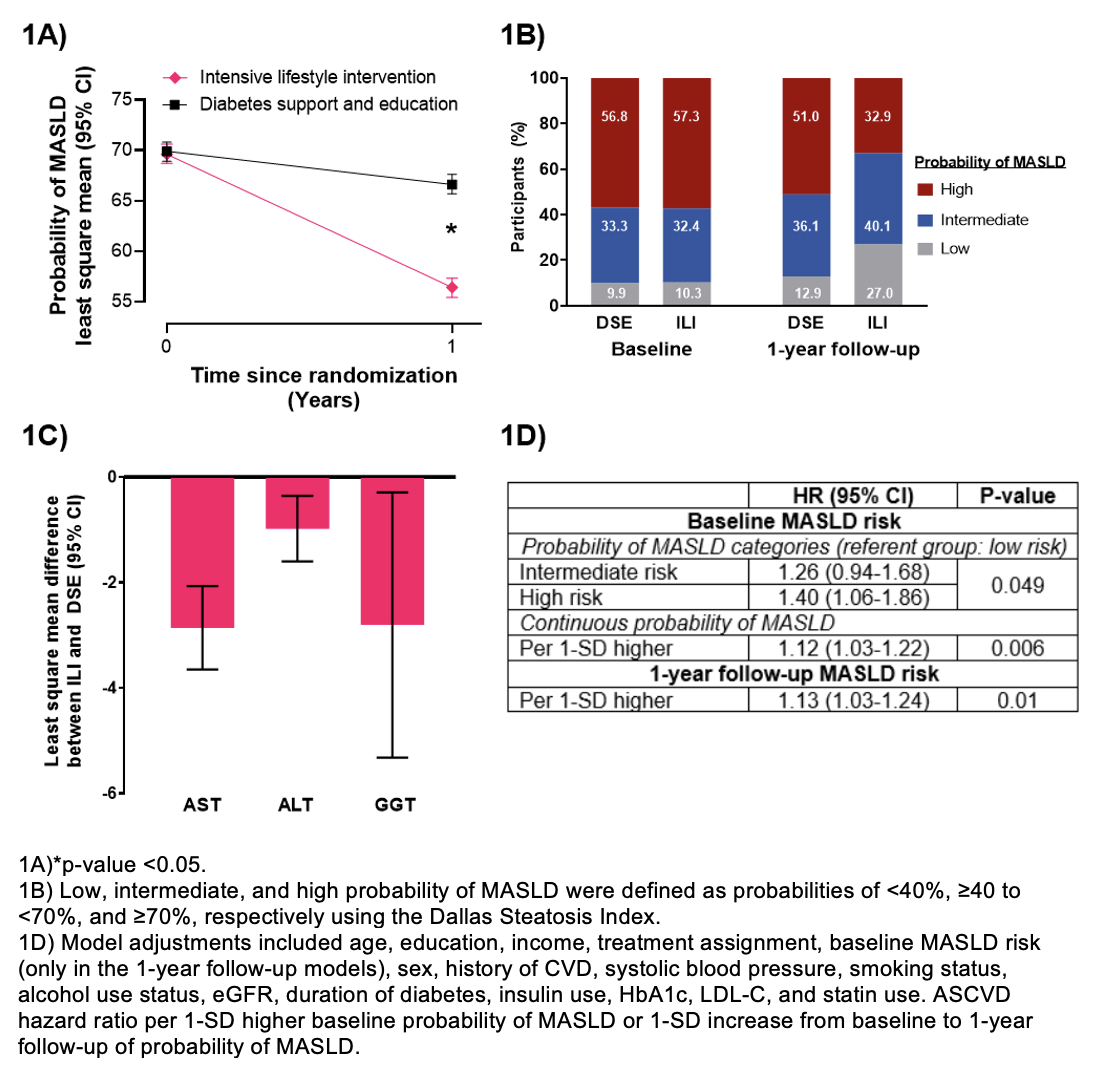
Results: The present study included 3,938 participants with AST, ALT, and GGT data. Among 244 1H MRS studies, DSI demonstrated fair performance for predicting MASLD (C-statistic: 0.693) and was similar for the FSI at 0.688. The probability of MASLD was estimated in all study participants using the DSI. At 1-year of follow-up, the ILI (versus DSE) reduced the probability of MASLD by 10.3% (Figure 1A), decreased the proportion of participants with high probability of MASLD (≥70%) (Figure 1B), and reduced AST, ALT, and GGT (Figure 1C). In adjusted analyses, higher baseline probability of MASLD was significantly associated with higher risk of ASCVD (Figure 1D). Increase in probability of MASLD over 1-year follow-up was significantly associated with higher risk of ASCVD.
Conclusions: Among adults with T2D and overweight or obesity, an ILI targeting weight loss reduced the probability of MASLD as well as AST, ALT, and GGT. Higher baseline and longitudinal increases in the probability of MASLD were associated with ASCVD.
- Chunawala, Zainali ( University of Texas Southwestern , Dallas , Texas , United States )
- Butler, Javed ( Baylor Scott and White , Dallas , Texas , United States )
- Ballantyne, Christie ( BAYLOR COLLEGE MEDICINE , Houston , Texas , United States )
- Bertoni, Alain ( WAKE FOREST UNIV SCHOOL MED , Pfafftown , North Carolina , United States )
- Espeland, Mark ( Wake Forest School of Medicine , Winston-Salem , North Carolina , United States )
- De Lemos, James ( UT SOUTHWESTERN MEDICAL CTR , Dallas , Texas , United States )
- Pandey, Ambarish ( UT Southwestern Medical Center , Dallas , Texas , United States )
- Patel, Kershaw ( Houston Methodist Hospital , Houston , Texas , United States )
- Clark, Jeanne ( Rutgers Robert Wood Johnson Med Sch , New Brunswick , New Jersey , United States )
- Vanwagner, Lisa ( UT Southwestern Medical Center , Dallas , Texas , United States )
- Browning, Jeffrey ( UTSW Medical Center , Dallas , Texas , United States )
- Garcia, Katelyn ( Wake Forest Baptist Health , Winston-Salem , North Carolina , United States )
- Zannad, Faiez ( CVCT and Universite de Lorraine , Paris , France )
- Maruthur, Nisa ( John Hopkins , Baltimore , Maryland , United States )
- Sanyal, Arun ( Virginia Commonwealth University , Richmond , Virginia , United States )
Meeting Info:
Session Info:
Saturday, 11/08/2025 , 03:15PM - 04:30PM
Abstract Oral Session
More abstracts on this topic:
Nair Tiny, Jabbar P, Ray Saumitra, Hazra Prakash, Gupta Sushil, Joshi Ameya, Shaikh Shehla, B Jayagopal, Pandit Kaushik, Sharma D, Seshadri Krishna, S Sridhar
Association of Triglyceride Glucose-Related Parameters with All-cause mortality and Cardiovascular Disease in Non-alcoholic Fatty Liver Disease PatientsChen Yaqin, Zhang Yusha, Wang Fengjiao
More abstracts from these authors:
Patel Kershaw, Bertoni Alain, Espeland Mark, Pandey Ambarish, Chunawala Zainali, Segar Matthew, Garcia Katelyn, Ndumele Chiadi, Wang Thomas, Januzzi James, Butler Javed, Lam Carolyn
Associations of baseline and longitudinal changes in MASLD with risk of heart failure events in type 2 diabetes and overweight or obesity: the Look Action for Health in Diabetes (Look AHEAD) Trial Liver Ancillary StudyChunawala Zainali, Butler Javed, Ballantyne Christie, Bertoni Alain, Espeland Mark, De Lemos James, Pandey Ambarish, Patel Kershaw, Clark Jeanne, Vanwagner Lisa, Browning Jeffrey, Garcia Katelyn, Zannad Faiez, Maruthur Nisa, Sanyal Arun