Final ID: MP1865
Proof-of-Concept Viral Transduction in Human Living Myocardial Slices: Advancing Human Pre-Clinical Models for Cardiac Gene Therapy
Abstract Body (Do not enter title and authors here): Background
Cardiac gene therapy holds the potential to transform the treatment of inherited and acquired cardiovascular diseases by correcting the causative basis of disease. However, despite extensive research, progress has been hindered, primarily due to the limited translation of pre-clinical models to human clinical settings. More advanced, human-based pre-clinical platforms are urgently needed to bridge the gap from bench to bedside. Human living myocardial slices (LMS), are 3D tissue sections with native multicellularity, structure and function, providing a promising platform to address these challenges.
Aims
Our study aimed to develop a platform for viral transduction of cardiomyocytes in human LMS, to advance pre-clinical gene therapy research and accelerate cardiac nucleic acid drug development.
Methods
LMS prepared from failing and non-failing donor hearts were cultured at physiological preload in a specialized biomimetic culture chamber with continuous force monitoring. After 2-4 days in culture, adenovirus carrying a fluorescent probe (GFP or mCherry) with either a ubiquitous or a cardiac-specific promoter was applied directly to the LMS surface at varying multiplicities of infection (MOIs). Slice contractility was monitored throughout culture to assess virus and transgene effects on function. At culture endpoint (5-10 days post transduction), slices were characterized using isometric Frank-Starling experiments in a custom bioreactor, fixed and probed for protein expression with antibodies, visualized via confocal microscopy, and lysed for transgene protein expression analysis via western blotting.
Results
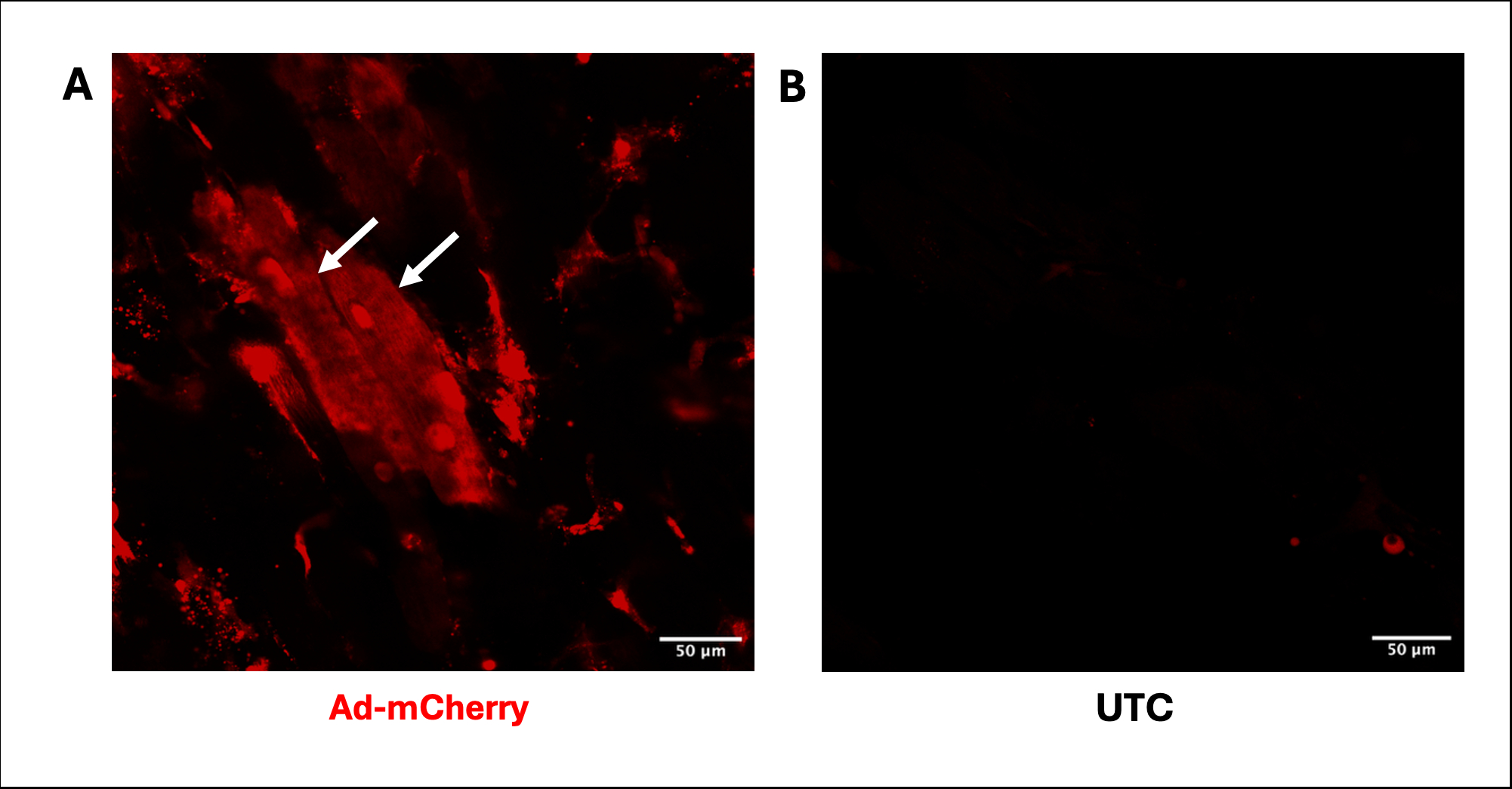
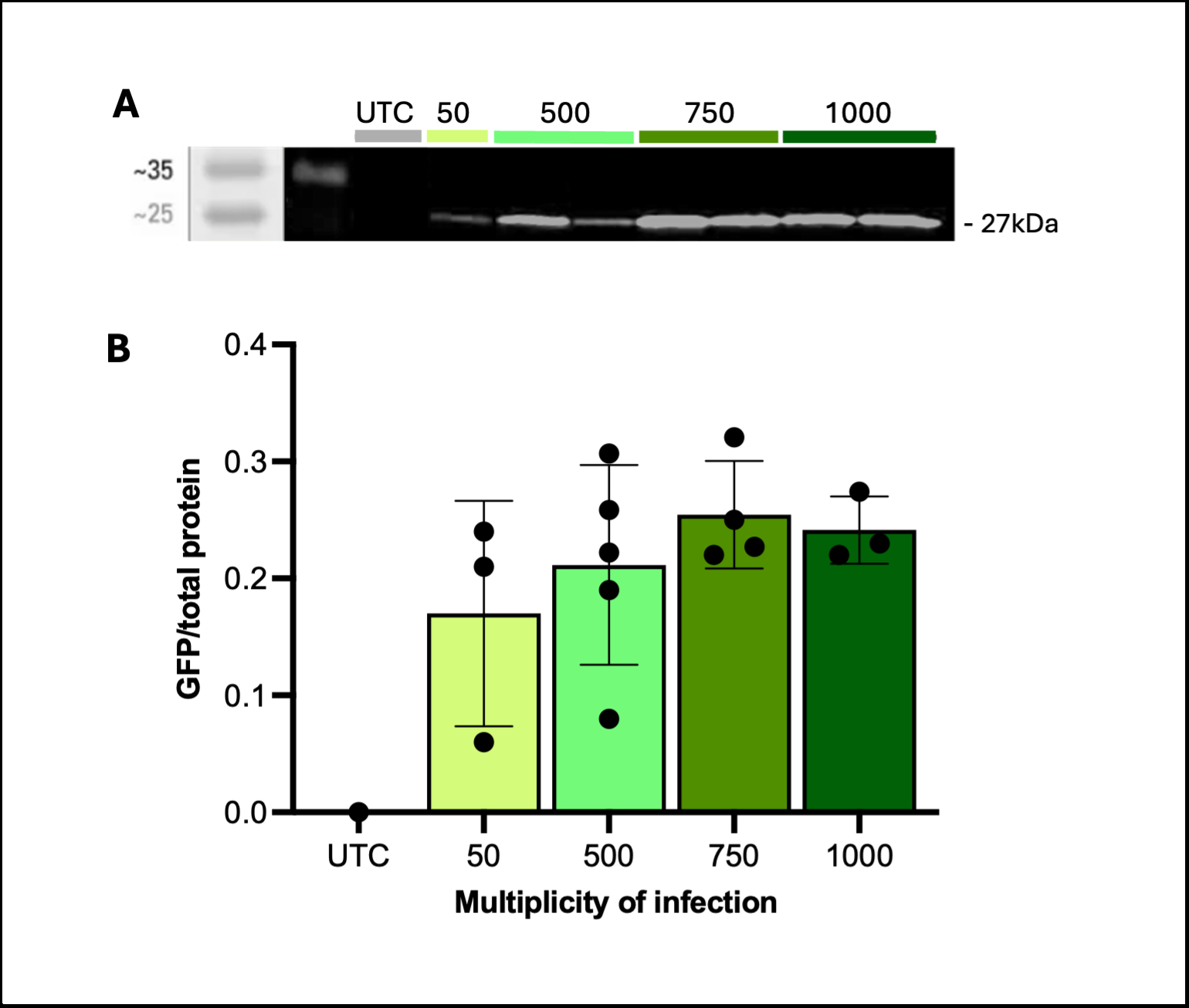
We demonstrate reproducible adenoviral transduction of GFP and mCherry in both cardiomyocytes and non-cardiomyocytes without compromising contractile function. An MOI-dependent increase in transduction and protein expression was observed, confirmed through confocal imaging (Figure 1) and western blot analysis (Figure 2).
Conclusion
This proof-of-concept study establishes feasibility of viral transduction of human LMS from explanted hearts, opening new avenues for translational heart research in the gene therapy space. Our expertise in LMS methodology positions us to develop this platform as a novel pre-clinical tool for genetic therapies, with future studies focusing on transducing failing human hearts with cardiac recovery-associated proteins.
Cardiac gene therapy holds the potential to transform the treatment of inherited and acquired cardiovascular diseases by correcting the causative basis of disease. However, despite extensive research, progress has been hindered, primarily due to the limited translation of pre-clinical models to human clinical settings. More advanced, human-based pre-clinical platforms are urgently needed to bridge the gap from bench to bedside. Human living myocardial slices (LMS), are 3D tissue sections with native multicellularity, structure and function, providing a promising platform to address these challenges.
Aims
Our study aimed to develop a platform for viral transduction of cardiomyocytes in human LMS, to advance pre-clinical gene therapy research and accelerate cardiac nucleic acid drug development.
Methods
LMS prepared from failing and non-failing donor hearts were cultured at physiological preload in a specialized biomimetic culture chamber with continuous force monitoring. After 2-4 days in culture, adenovirus carrying a fluorescent probe (GFP or mCherry) with either a ubiquitous or a cardiac-specific promoter was applied directly to the LMS surface at varying multiplicities of infection (MOIs). Slice contractility was monitored throughout culture to assess virus and transgene effects on function. At culture endpoint (5-10 days post transduction), slices were characterized using isometric Frank-Starling experiments in a custom bioreactor, fixed and probed for protein expression with antibodies, visualized via confocal microscopy, and lysed for transgene protein expression analysis via western blotting.
Results
We demonstrate reproducible adenoviral transduction of GFP and mCherry in both cardiomyocytes and non-cardiomyocytes without compromising contractile function. An MOI-dependent increase in transduction and protein expression was observed, confirmed through confocal imaging (Figure 1) and western blot analysis (Figure 2).
Conclusion
This proof-of-concept study establishes feasibility of viral transduction of human LMS from explanted hearts, opening new avenues for translational heart research in the gene therapy space. Our expertise in LMS methodology positions us to develop this platform as a novel pre-clinical tool for genetic therapies, with future studies focusing on transducing failing human hearts with cardiac recovery-associated proteins.
More abstracts on this topic:
A Suppression-and-Replacement Platform Identifies Pathogenic Variants in the LMNA-Encoded Lamin A/C Ig-Like Domain as Drivers of Nuclear Distortion and Aggregation
Huynh Trung, Kim Changsung, Tester David, Castrichini Matteo, Giudicessi John, Ackerman Michael
Metabolic Syndrome Alters cAMP Homeostasis and Contractile Function of CardiomyocytesPizzo Emanuele, Rota Marcello, Cervantes Daniel, Ripa Valentina, Jagana Vineeta, Ketkar Harshada, Singh Kanwardeep, Jacobson Jason, Jain Sudhir, Bisserier Malik