Final ID: 4369574
A Polypill Strategy for Lipid Lowering and Anti-Platelet Therapy After Acute Coronary Syndrome: A Pilot Randomized Controlled Trial
Abstract Body (Do not enter title and authors here): Background: Medication non-adherence following acute coronary syndrome (ACS) is a critical implementation gap in socioeconomically disadvantaged patients. The SECURE trial demonstrated benefits of polypill therapy in chronic CAD but excluded P2Y12 inhibitors and did not target socioeconomically disadvantaged populations with recent ACS.
Methods: In this open-label, two-center pilot RCT, adults within 30 days of ACS with stent placement were randomized 1:1 to once-daily polypill (aspirin 81mg, rosuvastatin 40mg, plus clopidogrel 75mg or prasugrel 10mg) versus usual care. Key endpoints were follow-up low-density lipoprotein cholesterol (LDL-C) and platelet reactivity, measured by impedance aggregometry (ohms [Ω]; lower = better inhibition), at 30 days. Patient-centered endpoints included treatment satisfaction (TSQM), adherence (MMAS-8), and quality of life (SAQ). Within-group changes from baseline to follow-up were compared, and treatment effects were assessed as differences least square means (LSM) using baseline-adjusted linear regression models.
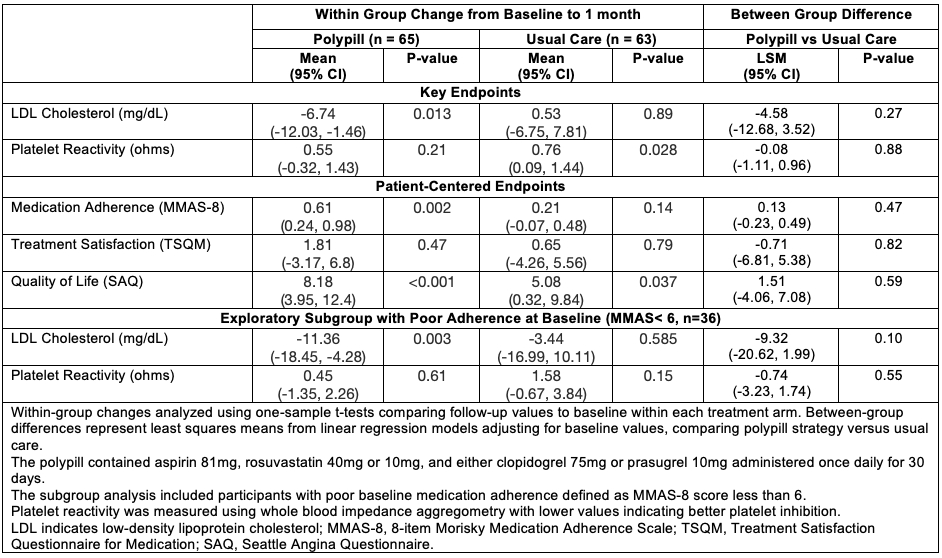
Results: Among 140 randomized participants (median age 58 years, 29% female, 14% Black, 63% Hispanic), 128 (91.4%) completed follow-up. Index ACS events were STEMI (29%), NSTEMI (51%), and unstable angina (19%). In the polypill arm, there was a significant LDL-C reduction (-6.74 mg/dL, p=0.013) with no change in platelet reactivity (+0.55Ω, p=0.21) from baseline to 30-day follow-up. In contrast, the usual care participants had no change in LDL-C and a significant worsening in platelet reactivity (0.76Ω, p=0.028). The between-group (polypill vs. usual care) differences were non-significant for LDL-C (LSM difference: -4.58mg/dL, p=0.27) and platelet reactivity (-0.08Ω, p=0.88). Between-group differences were non-significant for all patient-reported endpoints. In exploratory subgroup in those with poor baseline adherence, between-group LDL-C difference was numerically greater (-9.32 mg/dL, p=0.10). Composite ED/hospitalizations events were similar between polypill vs usual care (0.14 vs 0.19 events/participant, p=0.50).
Conclusions: This pilot RCT demonstrates feasibility of a polypill strategy incorporating P2Y12 inhibitors and statins among socioeconomically disadvantaged patients in the post-ACS period. Significant within-group improvements in LDL-C and adherence support the need for adequately powered longer-duration trials to evaluate sustained clinical benefits.
Methods: In this open-label, two-center pilot RCT, adults within 30 days of ACS with stent placement were randomized 1:1 to once-daily polypill (aspirin 81mg, rosuvastatin 40mg, plus clopidogrel 75mg or prasugrel 10mg) versus usual care. Key endpoints were follow-up low-density lipoprotein cholesterol (LDL-C) and platelet reactivity, measured by impedance aggregometry (ohms [Ω]; lower = better inhibition), at 30 days. Patient-centered endpoints included treatment satisfaction (TSQM), adherence (MMAS-8), and quality of life (SAQ). Within-group changes from baseline to follow-up were compared, and treatment effects were assessed as differences least square means (LSM) using baseline-adjusted linear regression models.
Results: Among 140 randomized participants (median age 58 years, 29% female, 14% Black, 63% Hispanic), 128 (91.4%) completed follow-up. Index ACS events were STEMI (29%), NSTEMI (51%), and unstable angina (19%). In the polypill arm, there was a significant LDL-C reduction (-6.74 mg/dL, p=0.013) with no change in platelet reactivity (+0.55Ω, p=0.21) from baseline to 30-day follow-up. In contrast, the usual care participants had no change in LDL-C and a significant worsening in platelet reactivity (0.76Ω, p=0.028). The between-group (polypill vs. usual care) differences were non-significant for LDL-C (LSM difference: -4.58mg/dL, p=0.27) and platelet reactivity (-0.08Ω, p=0.88). Between-group differences were non-significant for all patient-reported endpoints. In exploratory subgroup in those with poor baseline adherence, between-group LDL-C difference was numerically greater (-9.32 mg/dL, p=0.10). Composite ED/hospitalizations events were similar between polypill vs usual care (0.14 vs 0.19 events/participant, p=0.50).
Conclusions: This pilot RCT demonstrates feasibility of a polypill strategy incorporating P2Y12 inhibitors and statins among socioeconomically disadvantaged patients in the post-ACS period. Significant within-group improvements in LDL-C and adherence support the need for adequately powered longer-duration trials to evaluate sustained clinical benefits.
More abstracts on this topic:
Aspirin Versus P2Y12 Inhibitors as Monotherapy for Secondary Prevention in Coronary Artery Disease: A Systematic Review and Meta-analysis
Luna Leonardo, Barros Bruno
Acute Rhythm Change Reveals Atrial-Specific Metabolomic and Lipidomic Alterations in Atrial FibrillationKassar Ahmad, Bockus Lee, Haykal Romanos, Chamoun Nadia, Chahine Yaacoub, Al Yasiri Hala, Hensley Tori, Akoum Nazem