Final ID: Sa4030
Multi-Population Genome-Wide Association Study of Bicuspid Aortic Valve
Abstract Body (Do not enter title and authors here): Background: Bicuspid aortic valve (BAV) is a common congenital heart defect associated with serious cardiovascular complications, including thoracic aortic aneurysm and dissection (TAAD). Despite high heritability (~90%) and autosomal dominant inheritance, the genetic basis of BAV remains largely unknown.
Methods: To investigate the genetic architecture of BAV, we leveraged electronic health record data linked to imputed genotyping from the VA Million Veteran Program (MVP). We developed and validated a Natural Language Processing (NLP) algorithm to extract valve leaflet morphology from echocardiographic reports and identify MVP participants with definitive BAV. We then conducted a multi-population genome-wide association study (GWAS) comparing individuals with BAV (n = 9,571) to all other MVP participants (n = 631,091) across European, African, Admixed American, and East Asian populations. Lead variants were mapped to nearby genes, followed by pathway enrichment analysis. To assess shared genetic architecture between BAV and TAAD, we calculated genetic correlation using LD score regression and performed colocalization analysis to identify shared causal loci.
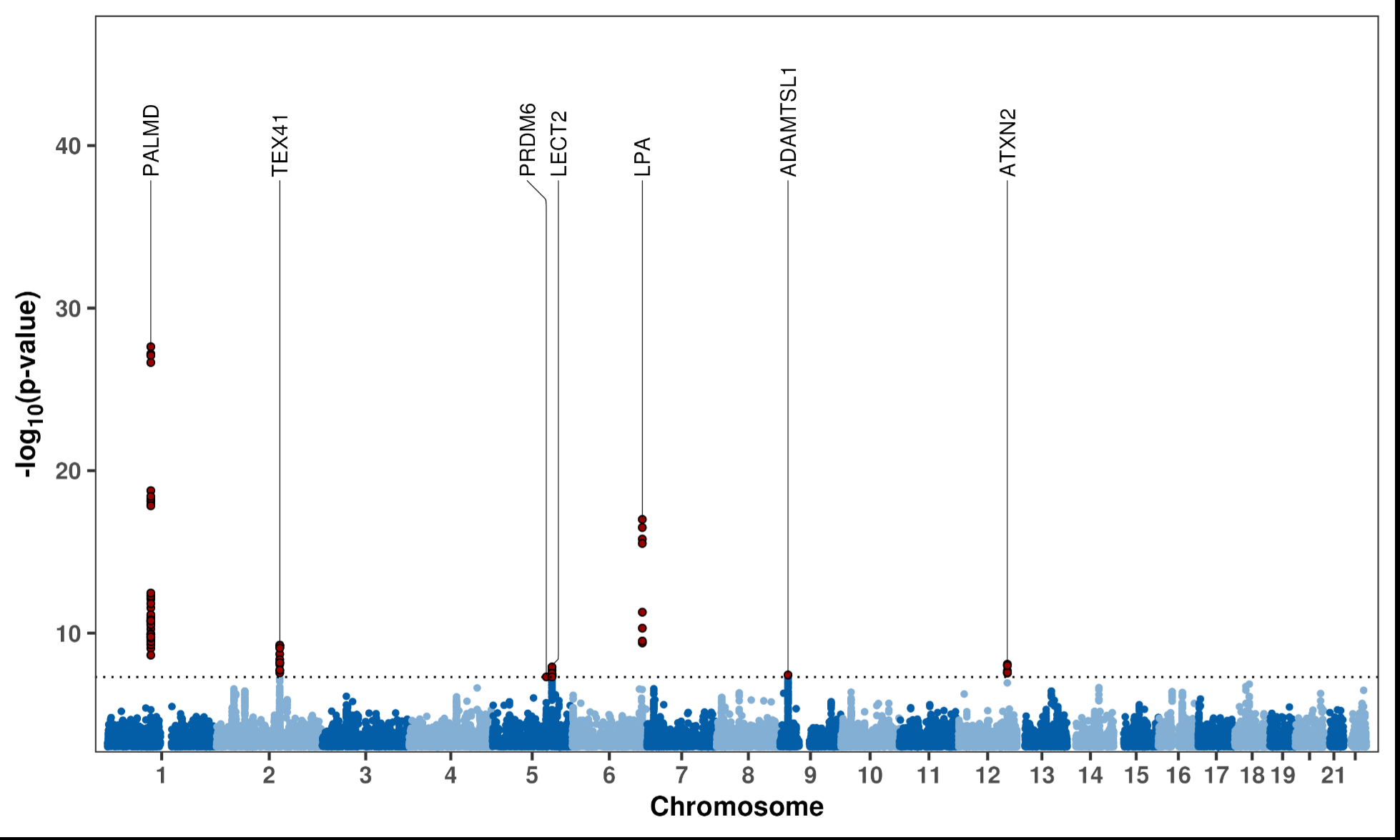
Results: Our NLP algorithm achieved high recall (0.955) and precision (0.984), identifying 655,762 reports (4.5%) and 83,704 patients (2.4%) with definitive BAV among 3.5 million Veterans. Of these, 9,571 had genotyping data and were included in the GWAS. Meta-analysis revealed seven loci reaching genome-wide significance (P < 5 × 10-8), including two known (PALMD, TEX41) and five novel loci (PRDM6, LECT2, LPA, ADAMTSL1, ATXN2) (Figure 1). Enriched pathways included extracellular matrix organization, O-linked glycosylation, smooth muscle cell differentiation, and lysine degradation. BAV showed significant genetic correlation with TAAD (rg = 0.45; P = 5.64 × 10-6). Colocalization analysis revealed seven shared loci between BAV and TAAD, with the strongest shared signal near PRDM6 (posterior probability ≈ 0.52). Notably, PRDM6, which regulates smooth muscle cell differentiation, was previously implicated in a TAAD GWAS, reinforcing the possible genetic overlap between these conditions.
Conclusions: Our findings identify novel candidate genes associated with BAV and support a shared genetic architecture with TAAD, providing new insights into the pathogenesis of BAV and related aortopathies while highlighting targets for future investigation.
Methods: To investigate the genetic architecture of BAV, we leveraged electronic health record data linked to imputed genotyping from the VA Million Veteran Program (MVP). We developed and validated a Natural Language Processing (NLP) algorithm to extract valve leaflet morphology from echocardiographic reports and identify MVP participants with definitive BAV. We then conducted a multi-population genome-wide association study (GWAS) comparing individuals with BAV (n = 9,571) to all other MVP participants (n = 631,091) across European, African, Admixed American, and East Asian populations. Lead variants were mapped to nearby genes, followed by pathway enrichment analysis. To assess shared genetic architecture between BAV and TAAD, we calculated genetic correlation using LD score regression and performed colocalization analysis to identify shared causal loci.
Results: Our NLP algorithm achieved high recall (0.955) and precision (0.984), identifying 655,762 reports (4.5%) and 83,704 patients (2.4%) with definitive BAV among 3.5 million Veterans. Of these, 9,571 had genotyping data and were included in the GWAS. Meta-analysis revealed seven loci reaching genome-wide significance (P < 5 × 10-8), including two known (PALMD, TEX41) and five novel loci (PRDM6, LECT2, LPA, ADAMTSL1, ATXN2) (Figure 1). Enriched pathways included extracellular matrix organization, O-linked glycosylation, smooth muscle cell differentiation, and lysine degradation. BAV showed significant genetic correlation with TAAD (rg = 0.45; P = 5.64 × 10-6). Colocalization analysis revealed seven shared loci between BAV and TAAD, with the strongest shared signal near PRDM6 (posterior probability ≈ 0.52). Notably, PRDM6, which regulates smooth muscle cell differentiation, was previously implicated in a TAAD GWAS, reinforcing the possible genetic overlap between these conditions.
Conclusions: Our findings identify novel candidate genes associated with BAV and support a shared genetic architecture with TAAD, providing new insights into the pathogenesis of BAV and related aortopathies while highlighting targets for future investigation.
More abstracts on this topic:
A Diagnostic Pitfall: Subclavian Stenosis Mimicking Severe Aortic Stenosis on Echocardiography"
Ezaldin Shady, Abdelsalam Mahmoud, Elsayed Omar, Lee Marciano
A Rare Cause of Recurrent Heart Failure Exacerbations After Transcatheter Aortic Valve Replacement: Ventricular Septal Defect and Significant Paravalvular LeakMedina Jesse, Vincent Louis, Rodriguez Ferreira Esteban, Spence-miller Shanice, Fernandez Joel, Colombo Rosario, Calfa Marian