Final ID: Sa4024
Gene Editing in Congenital Heart Disease Models: A 15-Year Scoping Review of CRISPR/Cas9 Applications in Human and Animal Systems
Abstract Body (Do not enter title and authors here): Background:
Congenital heart disease (CHD) is the most common birth defect globally, yet its genetic underpinnings remain incompletely understood. Since the introduction of CRISPR/Cas9, gene editing has emerged as a transformative tool for modeling and potentially treating CHD. However, no consolidated review exists mapping the scope of CRISPR/Cas9-based applications in this domain.
Research Question:
What genes, models, and delivery strategies have been used in CRISPR/Cas9-based CHD research over the past 15 years, and what are the major outcomes and translational gaps?
Methods:
We conducted a scoping review following PRISMA-ScR guidelines. PubMed, Web of Science, and Embase were searched for studies published between 2010–2025 that utilized CRISPR/Cas9 in CHD-related gene editing. Inclusion criteria encompassed human-induced pluripotent stem cell (hiPSC) models and animal systems (e.g., mice, zebrafish) focused on either disease modeling or therapeutic correction of known CHD-associated genes.
Results:
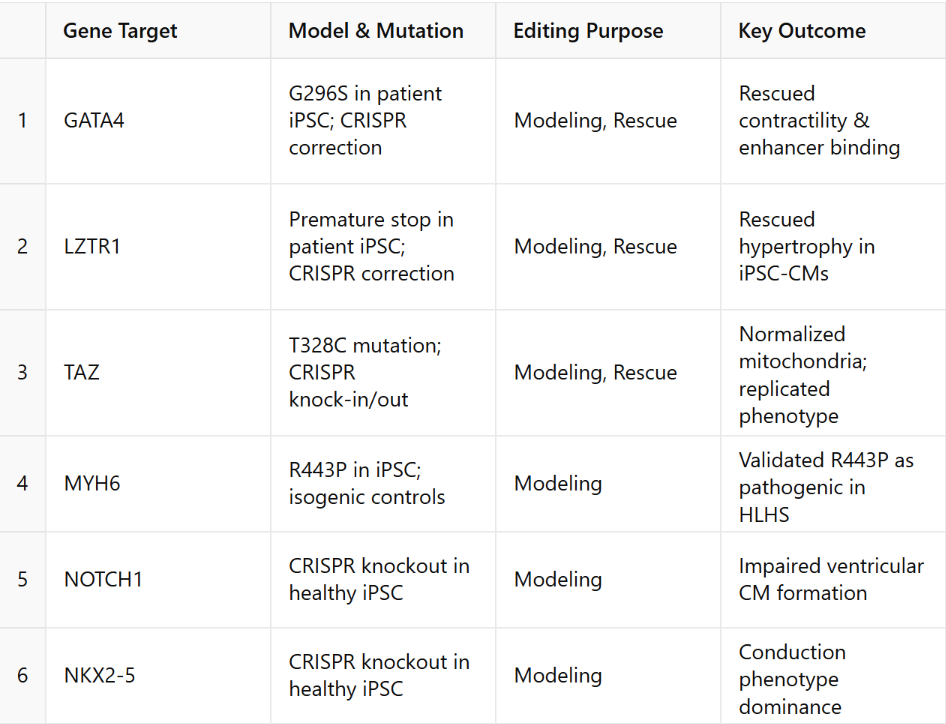
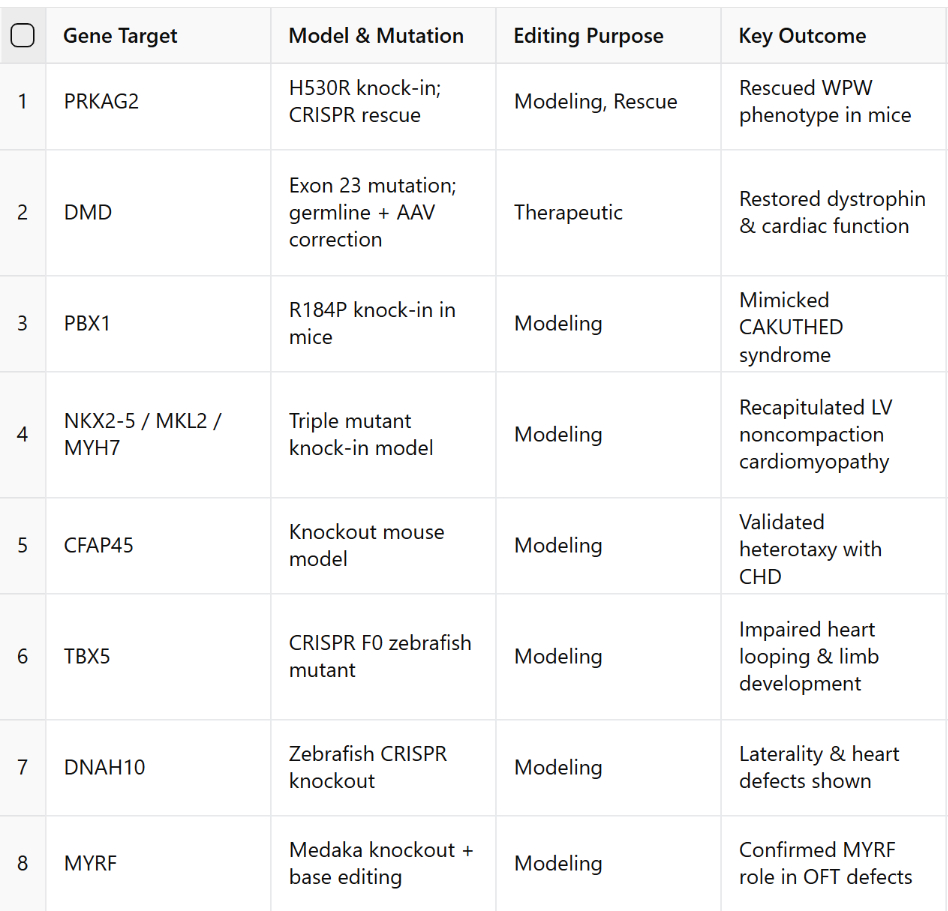
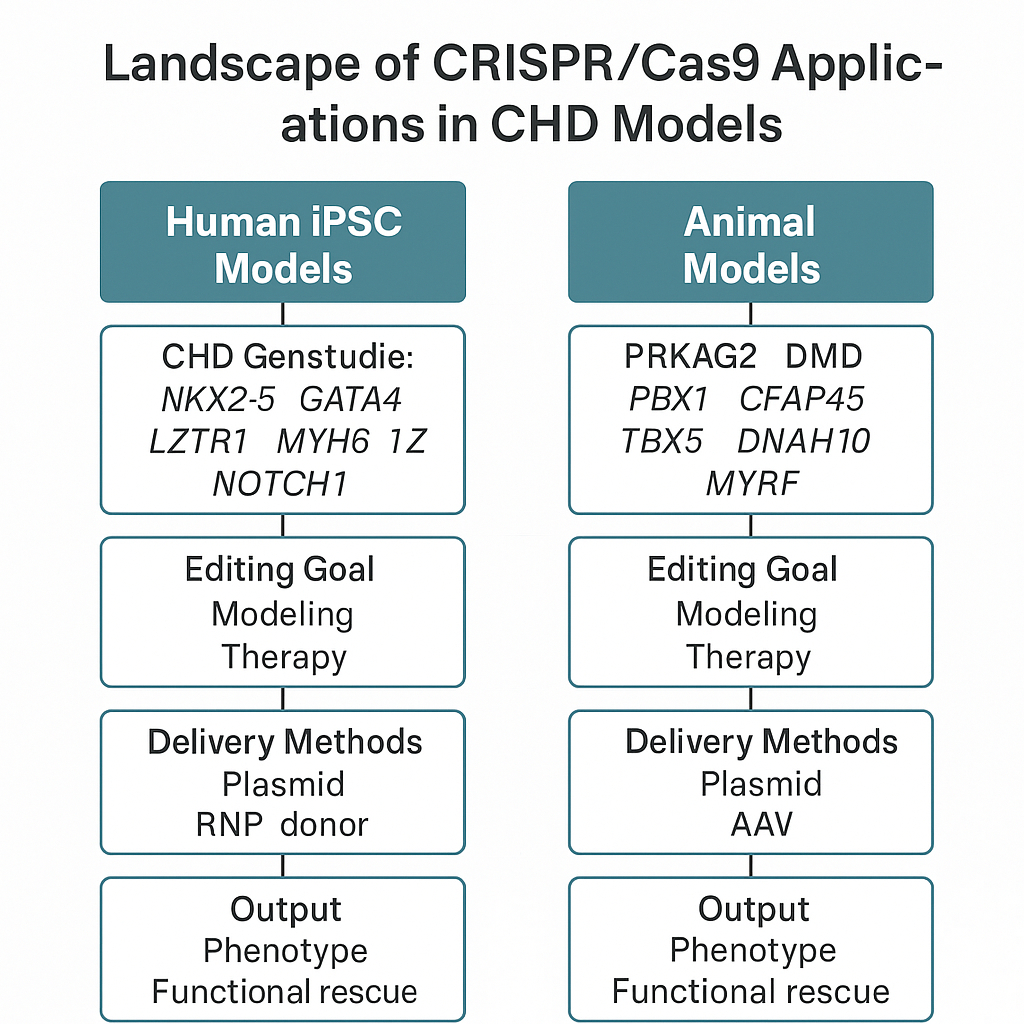
A total of 87 studies met inclusion criteria. In human hiPSC models, CRISPR was primarily used to introduce or correct mutations in key CHD genes (NKX2-5, GATA4, TBX5, MYH6, NOTCH1), enabling mechanistic insights and phenotypic rescue in cardiomyocytes. In animal models, CRISPR/Cas9 created disease-relevant knockouts or knock-ins across species such as mice, zebrafish, and medaka. Notably, therapeutic editing was successfully demonstrated in neonatal mice using AAV9-mediated somatic CRISPR to correct PRKAG2 and DMD mutations. However, few studies addressed polygenic inheritance, in utero delivery, or long-term safety. Tables 1 and 2 summarize the genes, models, editing purposes, delivery methods, and outcomes. A conceptual figure maps the landscape of CRISPR applications in CHD.
Conclusion:
CRISPR/Cas9 has revolutionized CHD research, enabling precise modeling and first steps toward somatic correction in preclinical systems. Nonetheless, significant translational barriers remain, including delivery challenges, modeling of complex genotypes, and ethical concerns with prenatal editing. Future studies integrating base editing, 3D cardiac organoids, and multiplex gene targeting may overcome current limitations. This scoping review identifies critical gaps and serves as a roadmap for accelerating genome-editing-based CHD therapeutics.
Congenital heart disease (CHD) is the most common birth defect globally, yet its genetic underpinnings remain incompletely understood. Since the introduction of CRISPR/Cas9, gene editing has emerged as a transformative tool for modeling and potentially treating CHD. However, no consolidated review exists mapping the scope of CRISPR/Cas9-based applications in this domain.
Research Question:
What genes, models, and delivery strategies have been used in CRISPR/Cas9-based CHD research over the past 15 years, and what are the major outcomes and translational gaps?
Methods:
We conducted a scoping review following PRISMA-ScR guidelines. PubMed, Web of Science, and Embase were searched for studies published between 2010–2025 that utilized CRISPR/Cas9 in CHD-related gene editing. Inclusion criteria encompassed human-induced pluripotent stem cell (hiPSC) models and animal systems (e.g., mice, zebrafish) focused on either disease modeling or therapeutic correction of known CHD-associated genes.
Results:
A total of 87 studies met inclusion criteria. In human hiPSC models, CRISPR was primarily used to introduce or correct mutations in key CHD genes (NKX2-5, GATA4, TBX5, MYH6, NOTCH1), enabling mechanistic insights and phenotypic rescue in cardiomyocytes. In animal models, CRISPR/Cas9 created disease-relevant knockouts or knock-ins across species such as mice, zebrafish, and medaka. Notably, therapeutic editing was successfully demonstrated in neonatal mice using AAV9-mediated somatic CRISPR to correct PRKAG2 and DMD mutations. However, few studies addressed polygenic inheritance, in utero delivery, or long-term safety. Tables 1 and 2 summarize the genes, models, editing purposes, delivery methods, and outcomes. A conceptual figure maps the landscape of CRISPR applications in CHD.
Conclusion:
CRISPR/Cas9 has revolutionized CHD research, enabling precise modeling and first steps toward somatic correction in preclinical systems. Nonetheless, significant translational barriers remain, including delivery challenges, modeling of complex genotypes, and ethical concerns with prenatal editing. Future studies integrating base editing, 3D cardiac organoids, and multiplex gene targeting may overcome current limitations. This scoping review identifies critical gaps and serves as a roadmap for accelerating genome-editing-based CHD therapeutics.
More abstracts on this topic:
A Multi-Population-First Approach Leveraging UK Biobank (UKBB) and All of Us (AoU) Datasets Reveals Higher Cardiomyopathy Variant Burden in Individuals with Myocarditis
Gurumoorthi Manasa, Khanji Mohammed, Munroe Patricia, Petersen Steffen, Landstrom Andrew, Chahal Anwar, Hesse Kerrick, Asatryan Babken, Shah Ravi, Sharaf Dabbagh Ghaith, Wolfe Rachel, Shyam Sundar Vijay, Mohiddin Saidi, Aung Nay
A Comparison of Characteristics and Outcomes in Patients with and without Adult Congenital Heart Disease Undergoing Catheter Ablation for Ventricular TachycardiaFutela Pragyat, Poddar Aastha, Kowlgi Gurukripa