Final ID: Sa4058
Electronegative VLDL Drives Maturity-Dependent Cellular Dysfunction on Human Cardiomyocytes
Abstract Body (Do not enter title and authors here): Background
Lipid overload-induced cardiomyocyte dysfunction is a key contributor to heart failure and other cardiometabolic diseases. Very-low-density lipoprotein (VLDL), the main triglyceride carrier, can be fractionated into five subfractions from V1 (least electronegative) to V5 (most electronegative, least abundant). While V5 has been linked to atherogenicity, its metabolic effect on cardiomyocytes remains poorly understood. These effects may be further modulated by the maturation state of the cells, influencing stress response. We hypothesize that electronegative VLDL subclasses induce distinct metabolic and functional responses in cardiomyocytes depending on their maturation state.
Methods
V1 and V5 fractions were isolated from the plasma of healthy blood donors. Human neonatal (NCMs) and adult cardiomyocytes (ACMs) were treated with PBS (control), and V1 or V5 at 10 or 20 µg/mL for 24 hours. Total cholesterol in cell media was quantified with a colorimetric assay. Apoptosis, intracellular ROS, and mitochondrial superoxide were assessed via tricolor fluorescence staining. Mitochondrial function was evaluated using the Seahorse Cell Mito Stress Test.
Results
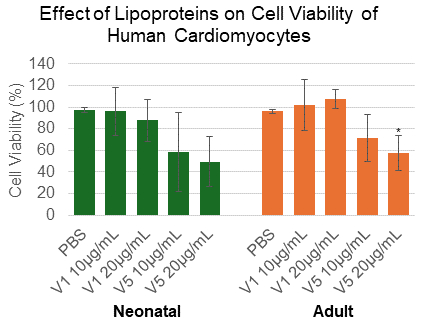
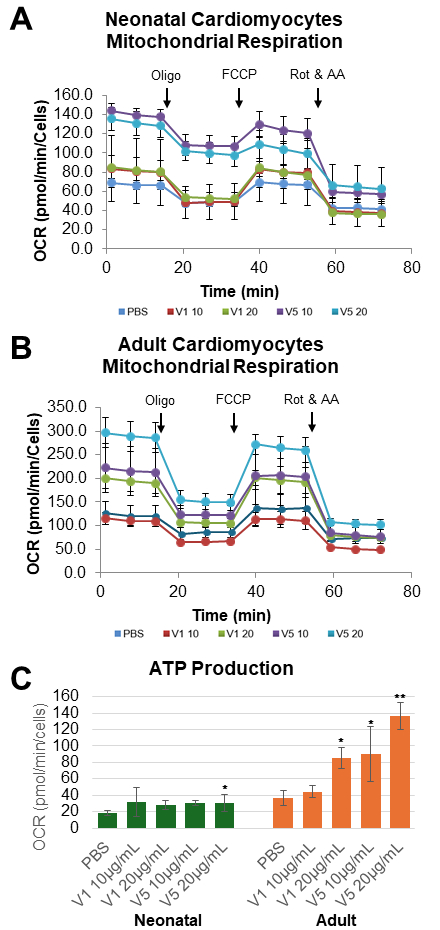
Effective lipoprotein uptake was confirmed in both NCMs and ACMs as evidenced by increased total cholesterol levels after V1 and V5 treatment. Apoptosis increased twofold in ACMs treated with 20 µg/mL V5 (p=0.006) but not in NCMs (Figure 1). V5 treatment elevated both total and mitochondrial ROS levels in NCMs, whereas ROS levels were reduced in ACMs, likely due to apoptotic cell loss. Seahorse analysis revealed that V5 induced significant mitochondrial dysfunction in NCMs, characterized by reduced ATP production and coupling efficiency, and increased proton leak. In ACMs, ATP production decreased with V5 at both concentrations, as well as with 20 µg/mL V1 (p<.01) (Figure 2).
Conclusion
This is the first report demonstrating that cardiomyocyte maturity significantly influences susceptibility to VLDL-induced metabolic stress, with adult cells primarily undergoing apoptosis and neonatal cells exhibiting extensive mitochondrial dysfunction. The differential effects of V1 and V5 subclasses, particularly at low concentrations, underscore the functional heterogeneity of lipoproteins. These maturity-dependent responses provide insight into how variations in lipoprotein metabolism may contribute to cardiac dysfunction in the context of developmental stage and cardiometabolic disease risk.
Lipid overload-induced cardiomyocyte dysfunction is a key contributor to heart failure and other cardiometabolic diseases. Very-low-density lipoprotein (VLDL), the main triglyceride carrier, can be fractionated into five subfractions from V1 (least electronegative) to V5 (most electronegative, least abundant). While V5 has been linked to atherogenicity, its metabolic effect on cardiomyocytes remains poorly understood. These effects may be further modulated by the maturation state of the cells, influencing stress response. We hypothesize that electronegative VLDL subclasses induce distinct metabolic and functional responses in cardiomyocytes depending on their maturation state.
Methods
V1 and V5 fractions were isolated from the plasma of healthy blood donors. Human neonatal (NCMs) and adult cardiomyocytes (ACMs) were treated with PBS (control), and V1 or V5 at 10 or 20 µg/mL for 24 hours. Total cholesterol in cell media was quantified with a colorimetric assay. Apoptosis, intracellular ROS, and mitochondrial superoxide were assessed via tricolor fluorescence staining. Mitochondrial function was evaluated using the Seahorse Cell Mito Stress Test.
Results
Effective lipoprotein uptake was confirmed in both NCMs and ACMs as evidenced by increased total cholesterol levels after V1 and V5 treatment. Apoptosis increased twofold in ACMs treated with 20 µg/mL V5 (p=0.006) but not in NCMs (Figure 1). V5 treatment elevated both total and mitochondrial ROS levels in NCMs, whereas ROS levels were reduced in ACMs, likely due to apoptotic cell loss. Seahorse analysis revealed that V5 induced significant mitochondrial dysfunction in NCMs, characterized by reduced ATP production and coupling efficiency, and increased proton leak. In ACMs, ATP production decreased with V5 at both concentrations, as well as with 20 µg/mL V1 (p<.01) (Figure 2).
Conclusion
This is the first report demonstrating that cardiomyocyte maturity significantly influences susceptibility to VLDL-induced metabolic stress, with adult cells primarily undergoing apoptosis and neonatal cells exhibiting extensive mitochondrial dysfunction. The differential effects of V1 and V5 subclasses, particularly at low concentrations, underscore the functional heterogeneity of lipoproteins. These maturity-dependent responses provide insight into how variations in lipoprotein metabolism may contribute to cardiac dysfunction in the context of developmental stage and cardiometabolic disease risk.
More abstracts on this topic:
Aberrant MicroRNA Expression May Underlie Cardiac Dysfunction in a rat model of Preeclampsia
Vaka Ramana, Campbell Nathan, Edwards Kristin, Hoang Ngoc, Zheng Baoying, Lamarca Babbette
Association Between Lp(a) and Cardiovascular Events in Individuals with Peripheral Artery Disease in the Mass General Brigham Lp(a) RegistryMcclintick Daniel, Gerhard Marie, Januzzi James, Di Carli Marcelo, Secemsky Eric, Bhatt Deepak, Blankstein Ron, Divakaran Sanjay, Biery David, Berman Adam, Besser Stephanie, Shiyovich Arthur, Singh Avinainder, Huck Daniel, Weber Brittany, Bonaca Marc