Final ID: Sa4072
Electronegative L5 Low-Density Lipoprotein Rapidly Impairs Endothelial Proliferation and Triggers Early Senescence
Abstract Body (Do not enter title and authors here): Background:
Electronegative low-density lipoprotein (LDL) subfractions, particularly L5, the most electronegative, have been implicated in endothelial cytotoxicity and atherogenesis. In contrast to L1, the least electronegative subfraction, L5 promotes oxidative stress and endothelial dysfunction.These alterations disturb vascular homeostasis, drive chronic inflammation, and contribute to atherosclerotic progression. However, the specific impact of L5 on endothelial cell proliferation and regeneration remains unclear.
Objective:
To investigate how the L5 LDL subfraction affects endothelial cell proliferation after short-term exposure.
Methods:
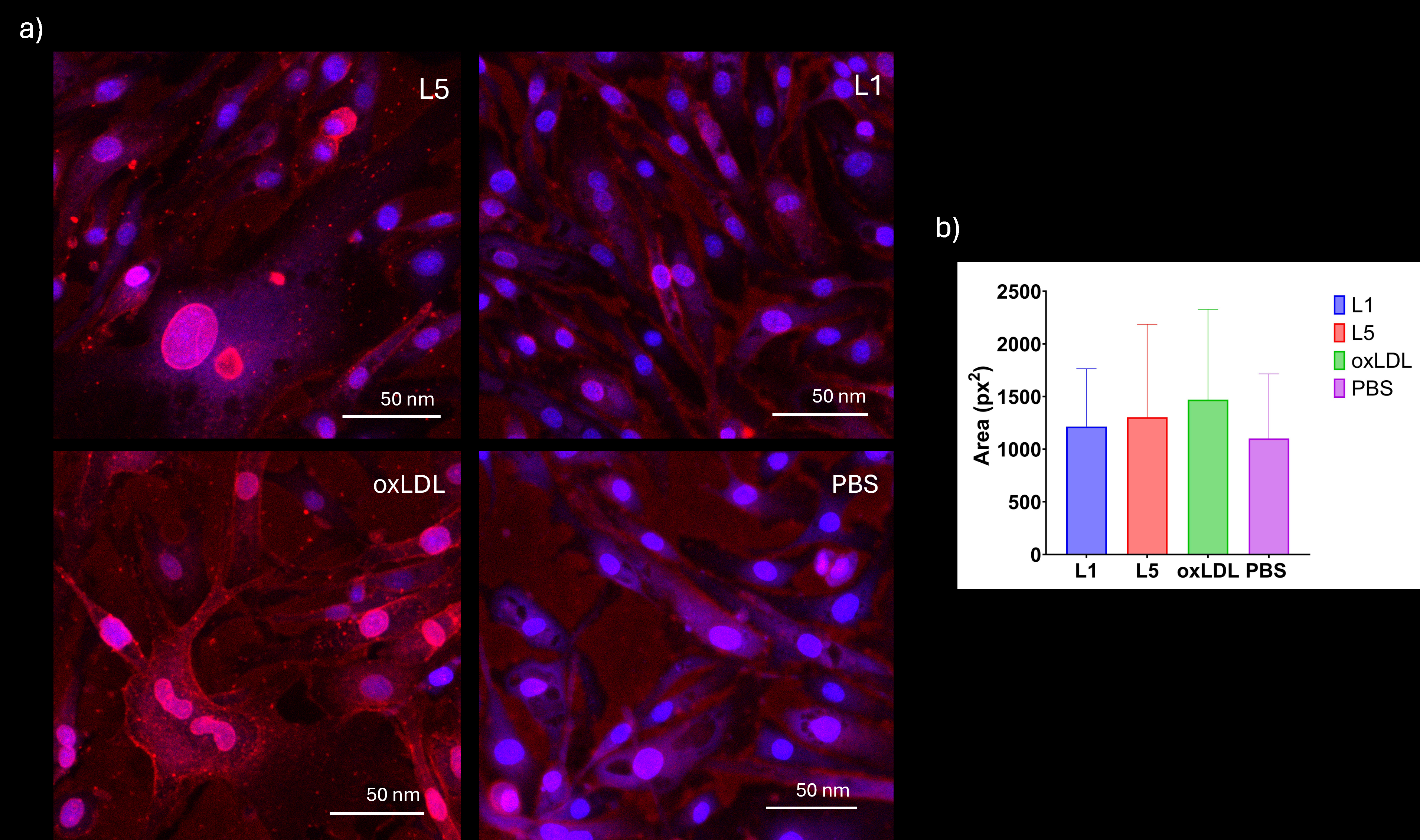
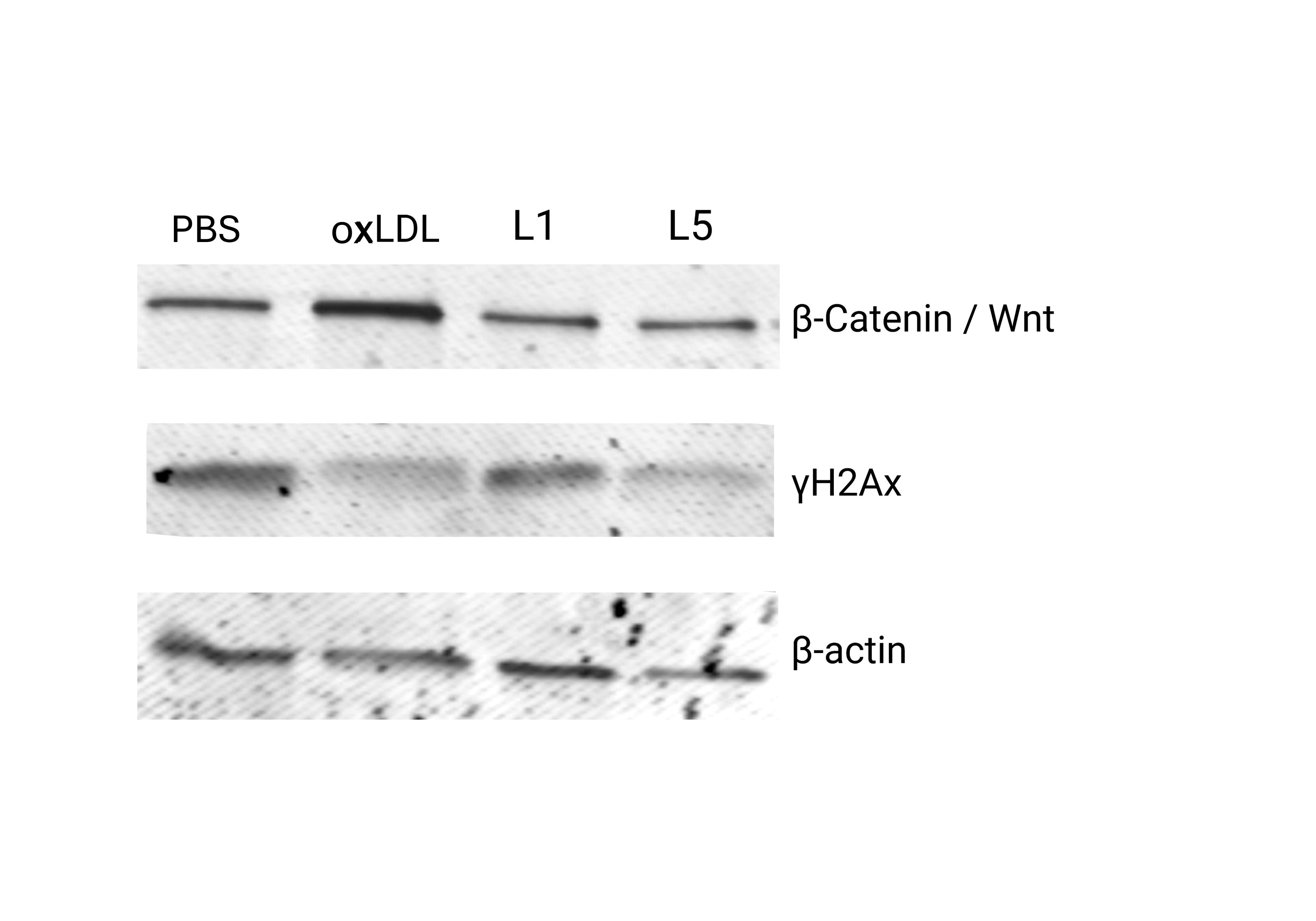
Bovine aortic endothelial cells (BAECs) were treated for 6 hours with 25 µg/mL of L1, L5, or oxidized LDL, and PBS. Membrane integrity and cellular morphology were assessed using wheat germ agglutinin (WGA) staining, while nuclear area was evaluated via DAPI staining. Apoptosis was quantified using Annexin V/PI staining by flow cytometry. Cell cycle analysis was conducted via PI staining. Western blotting was performed to assess protein expression levels of γH2AX (DNA damage marker) and β-catenin (Wnt signaling and endothelial injury marker).
Results:
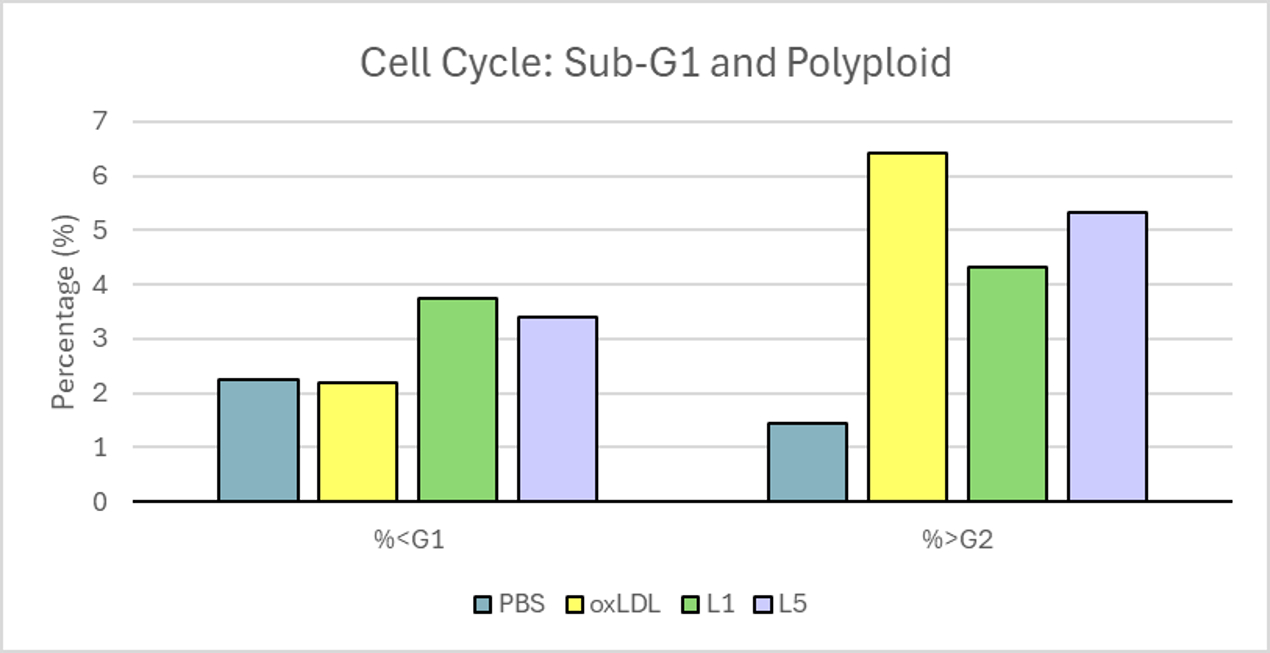
L5 treatment led to mild membrane disruption and abnormal nuclear morphology, with a nuclear area increase of 18% compared to PBS. While apoptosis and cell cycle differences were not statistically significant at 6 hours, an increased sub-G1 population suggested apoptotic activity in L5- and oxLDL-treated cells. Polyploid nuclei were 6 times more frequent in L5 group versus PBS controls. DNA damage, indicated by γH2AX expression, was decreased after L5 and oxLDL exposure. However, β-catenin levels remained unchanged at this early time point. L5 also presented polyploid accumulation, suggesting cell cycle arrest and initiation of senescence.
Conclusion:
This study shows that short-term exposure to L5 low-density lipoprotein disrupts endothelial proliferation by promoting nuclear abnormalities, apoptosis, and polyploidy, hallmarks of senescence. These effects may impair vascular regeneration and contribute to vascular dysfunction and myocardial ischemia. Our findings provide insight into how LDL electronegativity increases cardiovascular risk independent of total lipid levels. Future studies will explore long-term effects and protective strategies against L5-induced endothelial injury and potential endothelial-to-mesenchymal transition.
Electronegative low-density lipoprotein (LDL) subfractions, particularly L5, the most electronegative, have been implicated in endothelial cytotoxicity and atherogenesis. In contrast to L1, the least electronegative subfraction, L5 promotes oxidative stress and endothelial dysfunction.These alterations disturb vascular homeostasis, drive chronic inflammation, and contribute to atherosclerotic progression. However, the specific impact of L5 on endothelial cell proliferation and regeneration remains unclear.
Objective:
To investigate how the L5 LDL subfraction affects endothelial cell proliferation after short-term exposure.
Methods:
Bovine aortic endothelial cells (BAECs) were treated for 6 hours with 25 µg/mL of L1, L5, or oxidized LDL, and PBS. Membrane integrity and cellular morphology were assessed using wheat germ agglutinin (WGA) staining, while nuclear area was evaluated via DAPI staining. Apoptosis was quantified using Annexin V/PI staining by flow cytometry. Cell cycle analysis was conducted via PI staining. Western blotting was performed to assess protein expression levels of γH2AX (DNA damage marker) and β-catenin (Wnt signaling and endothelial injury marker).
Results:
L5 treatment led to mild membrane disruption and abnormal nuclear morphology, with a nuclear area increase of 18% compared to PBS. While apoptosis and cell cycle differences were not statistically significant at 6 hours, an increased sub-G1 population suggested apoptotic activity in L5- and oxLDL-treated cells. Polyploid nuclei were 6 times more frequent in L5 group versus PBS controls. DNA damage, indicated by γH2AX expression, was decreased after L5 and oxLDL exposure. However, β-catenin levels remained unchanged at this early time point. L5 also presented polyploid accumulation, suggesting cell cycle arrest and initiation of senescence.
Conclusion:
This study shows that short-term exposure to L5 low-density lipoprotein disrupts endothelial proliferation by promoting nuclear abnormalities, apoptosis, and polyploidy, hallmarks of senescence. These effects may impair vascular regeneration and contribute to vascular dysfunction and myocardial ischemia. Our findings provide insight into how LDL electronegativity increases cardiovascular risk independent of total lipid levels. Future studies will explore long-term effects and protective strategies against L5-induced endothelial injury and potential endothelial-to-mesenchymal transition.
More abstracts on this topic:
A Novel Role for Lipoprotein(a) in Potentiating Neutrophil Extracellular Trap Formation
Mouawad Sahar, Boffa Michael, Koschinsky Marlys
Aspirin Use and Cardiovascular Disease Incidence in Adults with High Lipoprotein(a): A Multi-Cohort StudyColantonio Lisandro, Bittner Vera, Wang Zhixin, Ghazi Lama, Alanaeme Chibuike, Christenson Ashley, Dubal Medha, Malick Waqas, Levitan Emily, Rosenson Robert