Final ID: MP2450
Rationale and Design of A Pragmatic Clinical Trial of the WE BEAT Well-Being Education Program in Adolescent Congenital Heart Disease: A Pediatric Heart Network (PHN) Study
Research Questions: The WE BEAT CHD Study aims to: 1) evaluate the effectiveness of the 5-week WE BEAT telemedicine intervention compared to usual care on self-reported resiliency and psychosocial outcomes including depression, anxiety, and quality of life, 2) determine associations between psychosocial survey data and cardiac outcomes including composite event score, heart failure, and metabolic health, and 3) establish a biorepository to associate psychosocial data with stress-related biomarkers, including stress cortisol, inflammatory biomarkers, and epigenetic aging.
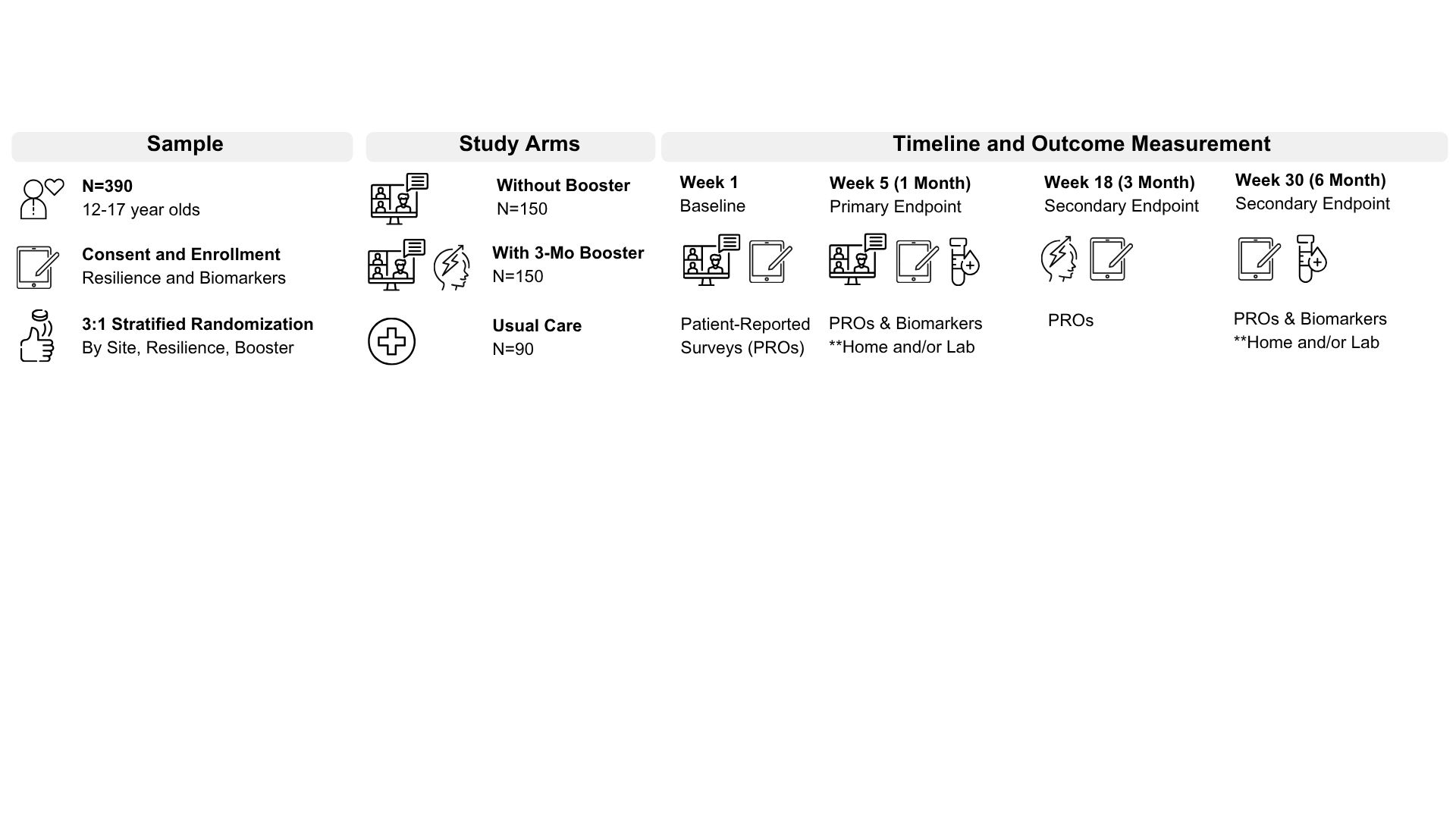
Approach: In this 2-arm, 2-staged stratified randomized, parallel, multi-center pragmatic trial (Figure 1), we seek to recruit 390 English or Spanish speaking 12-17-year-olds with moderately or severely complex CHD across ~15 PHN core and auxiliary sites. The intervention arm will include 300 participants and half will be randomized to complete a booster session. Study design was informed by a successful pilot study, patient/family input, and pragmatic clinical trial design. Five 45-minute intervention sessions and study surveys completed at baseline, 5, 18 and 30 weeks will be done remotely via video conferencing and a study mobile app. Home collection of biospecimen samples will be available. Study duration is estimated to be 5 years.
Results: The primary outcome will be change in participant self-reported resiliency as measured by the Connor-Davidson Resilience Scale© from baseline to Week 5, with secondary endpoints of change in resiliency from baseline to Weeks 18 and 30.
Conclusion: This study addresses the need for promotive mental-health-focused interventions for adolescents with CHD. The pragmatic trial design intends to bolster successful recruitment and ease of translation to clinical practice. Secondary aims position the trial for mechanistic investigations and future ancillary studies.
- Cousino, Melissa ( UNIVERSITY OF MICHIGAN , Ann Arbor , Michigan , United States )
- De Ferranti, Sarah ( Boston Children's Hospital , Boston , Massachusetts , United States )
- Fung, Amy ( CHILDRENS HOSPITAL COLORADO , Aurora , Colorado , United States )
- Graham, Eric ( Medical University of South Carolina , Charleston , South Carolina , United States )
- Kelly, Sarah ( CHILDRENS HOSPITAL COLORADO , Aurora , Colorado , United States )
- Kochilas, Lazaros ( EMORY UNIVERSITY , Atlanta , Georgia , United States )
- Lorenzi Quigley, Lauren ( UMPC , Pittsburgh , Pennsylvania , United States )
- May, Lindsay ( University of Utah , Salt Lake City , Utah , United States )
- Miller, Julie ( Carelon Research , Newton , Massachusetts , United States )
- Ybarra, Marion ( Washington University , St. Louis , Missouri , United States )
- Russell, Mark ( University of Michigan , Ann Arbor , Michigan , United States )
- Schumacher, Kurt ( UNIVERSITY OF MICHIGAN , Ann Arbor , Michigan , United States )
- Richmond, Marc ( COLUMBIA UNIVERSITY MSCHONY , New York , New York , United States )
- Miyamoto, Shelley ( CHILDRENS HOSPITAL COLORADO , Aurora , Colorado , United States )
- Teng, Jessica ( Carelon Research , Newton , Massachusetts , United States )
- Zak, Victor ( Carelon Research , Newton , Massachusetts , United States )
- Batazzi, Adriana ( University of Michigan , Ann Arbor , Michigan , United States )
- Bezold, Louis ( University of Kentucky , Lexington , Kentucky , United States )
- Bucholz, Emily ( University of Colorado Denver , Aurora , Colorado , United States )
- Burns, Kristin ( NHLBI/NIH , Bethesda , Maryland , United States )
- Crister, Paul ( Cincinatti Children's , Cincinatti , Ohio , United States )
Meeting Info:
Session Info:
Monday, 11/10/2025 , 12:15PM - 01:30PM
Moderated Digital Poster Session
More abstracts on this topic:
Ardakani Jad, Rajagopalan Sanjay, Maddock Jay E, Nasir Khurram, Al-kindi Sadeer, Dong Weichuan, Shahid Izza, Gullapelli Rakesh, Bose Budhaditya, Zhang Tong, Nicolas Juan, Javed Zulqarnain, Chen Zhuo
Characterizing Conventional and Expanded Adverse Childhood Experiences’ Effect on Mental Health in Black Men with Unideal Cardiovascular Health: Black ImpactAppana Bhavya, Kaur Pardeep, Saani Awal, Gregory John, Ortiz Robin, Joseph Joshua, Nolan Timiya, Zhao Songzhu, Grant Jeremy, Brock Guy, Wilson Amani, Lartey Kwame, Ojembe Nnanna, Adongo Jessica
More abstracts from these authors:
Jacobsen Roni, Ginde Salil, Schumacher Kurt, Miyamoto Shelley, Kelly Sarah, Sauceda Heidi, Payan Marisa, Schofield Samuel, Canter Charles, Ybarra Aecha, Connor Brynn, Earing Michael
WE CHATT: A Pilot Study to Improve Physician-Youth Communication and Medical Decision-Making in Pediatric Advanced Heart DiseaseVitale Carolyn, Smith Cynthia, Yu Sunkyung, Lowery Ray, Schumacher Kurt, Cousino Melissa