Final ID: MP2216
SELECT-LIFE: A non-interventional follow-up study investigating the long-term effects of semaglutide in participants from the SELECT trial
Research Questions: To evaluate long-term post-trial effects of once-weekly semaglutide vs placebo on MACE, obesity-related complications and metabolic-related outcomes.
Methods: SELECT assessed efficacy and safety of once-weekly semaglutide 2.4 mg vs placebo added to standard of care among participants with BMI ≥27 kg/m2 and established CVD without diabetes. SELECT-LIFE is an ongoing, prospective observational study in which participants self-report data via questionnaire every 6 months after SELECT completion, with a planned duration of 10 years. No trial drug was directly provided. We report results from the first three questionnaire cycles completed in November 2024 (median follow-up 16 months). Key endpoints: MACE (all-cause death, non-fatal myocardial infarction, or non-fatal stroke), time to type 2 diabetes (T2D) diagnosis, body weight, cancer incidence, knee replacement surgery, and use of a continuous positive airway pressure (CPAP) device for sleep apnea.
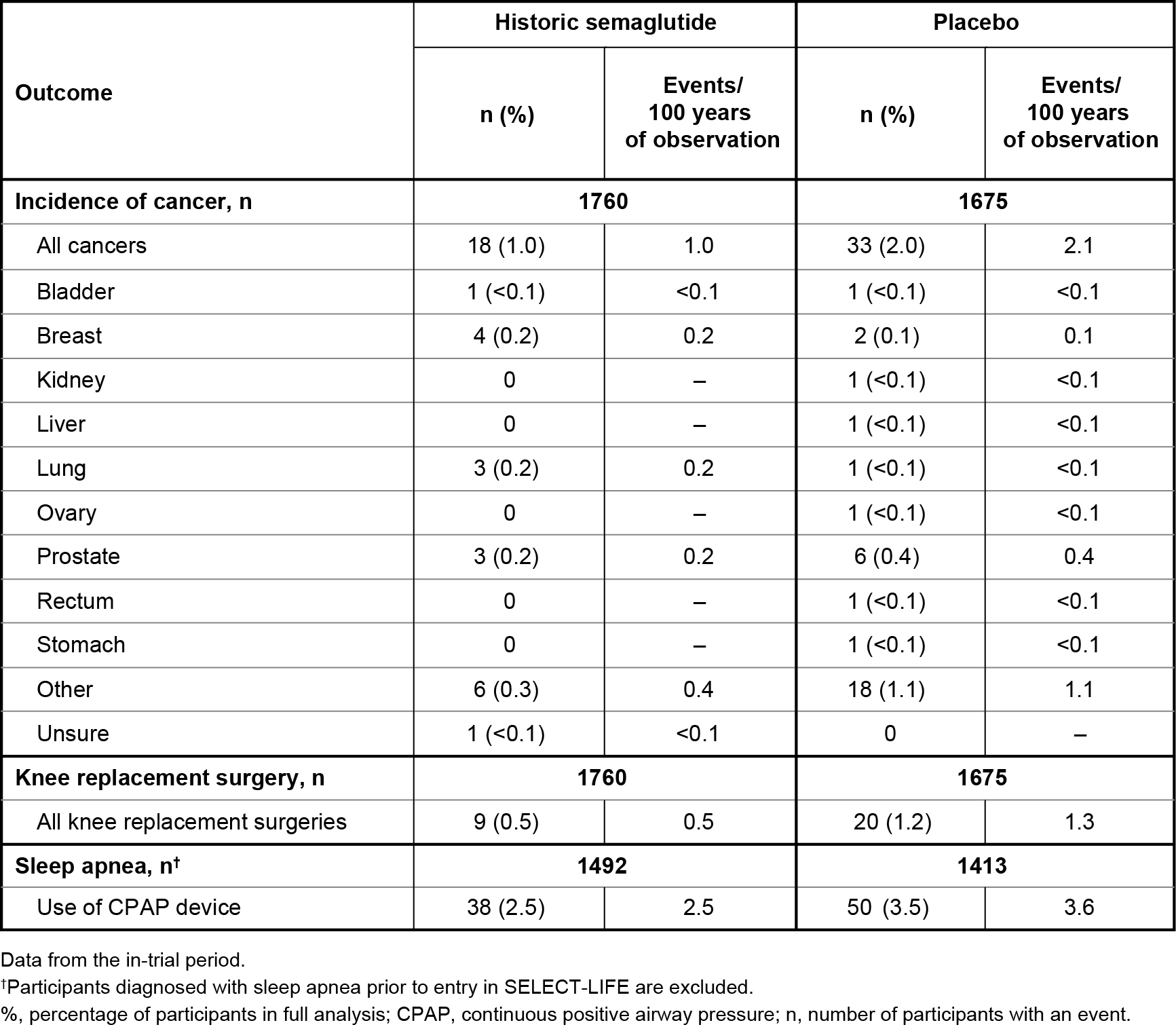
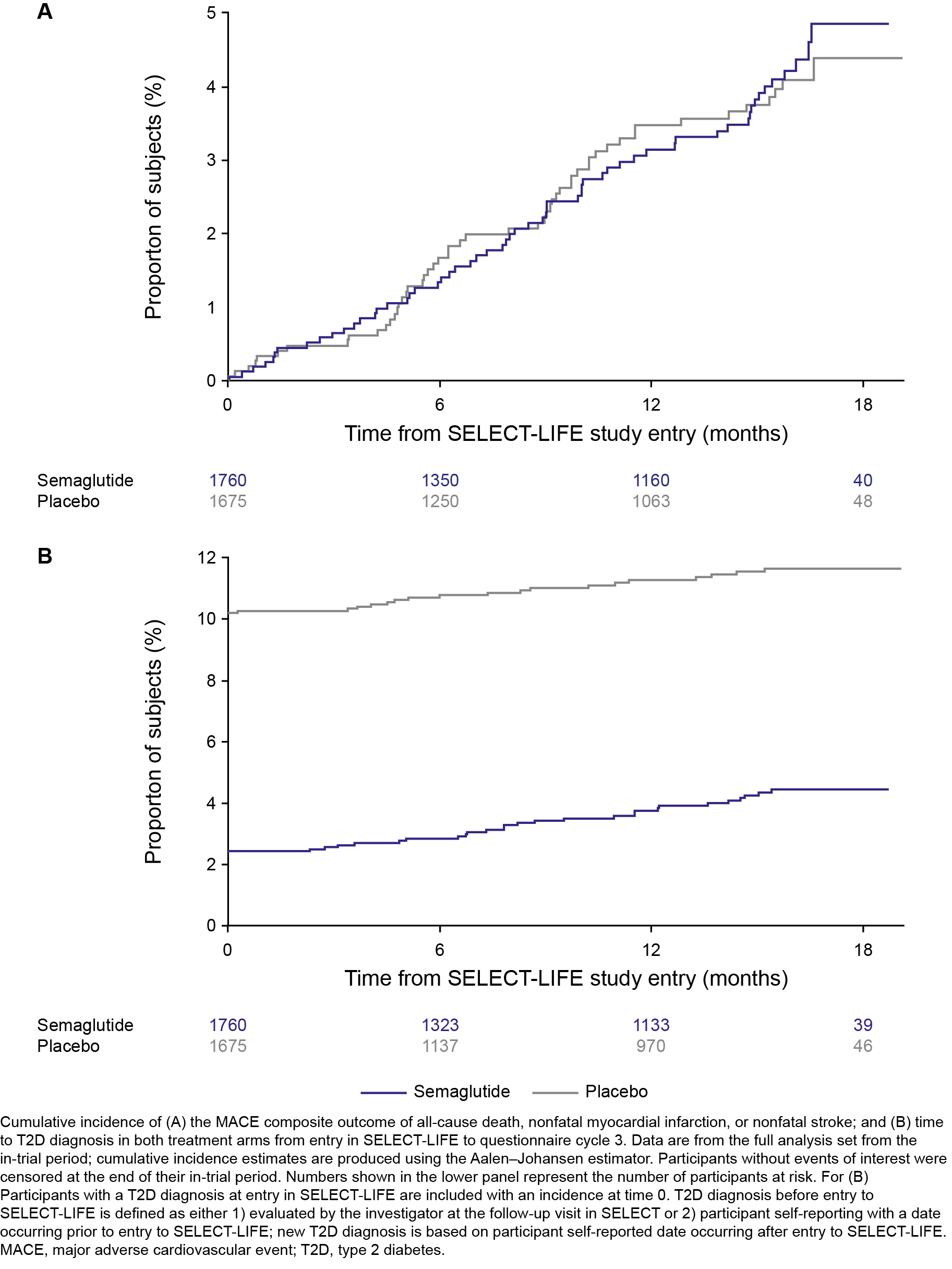
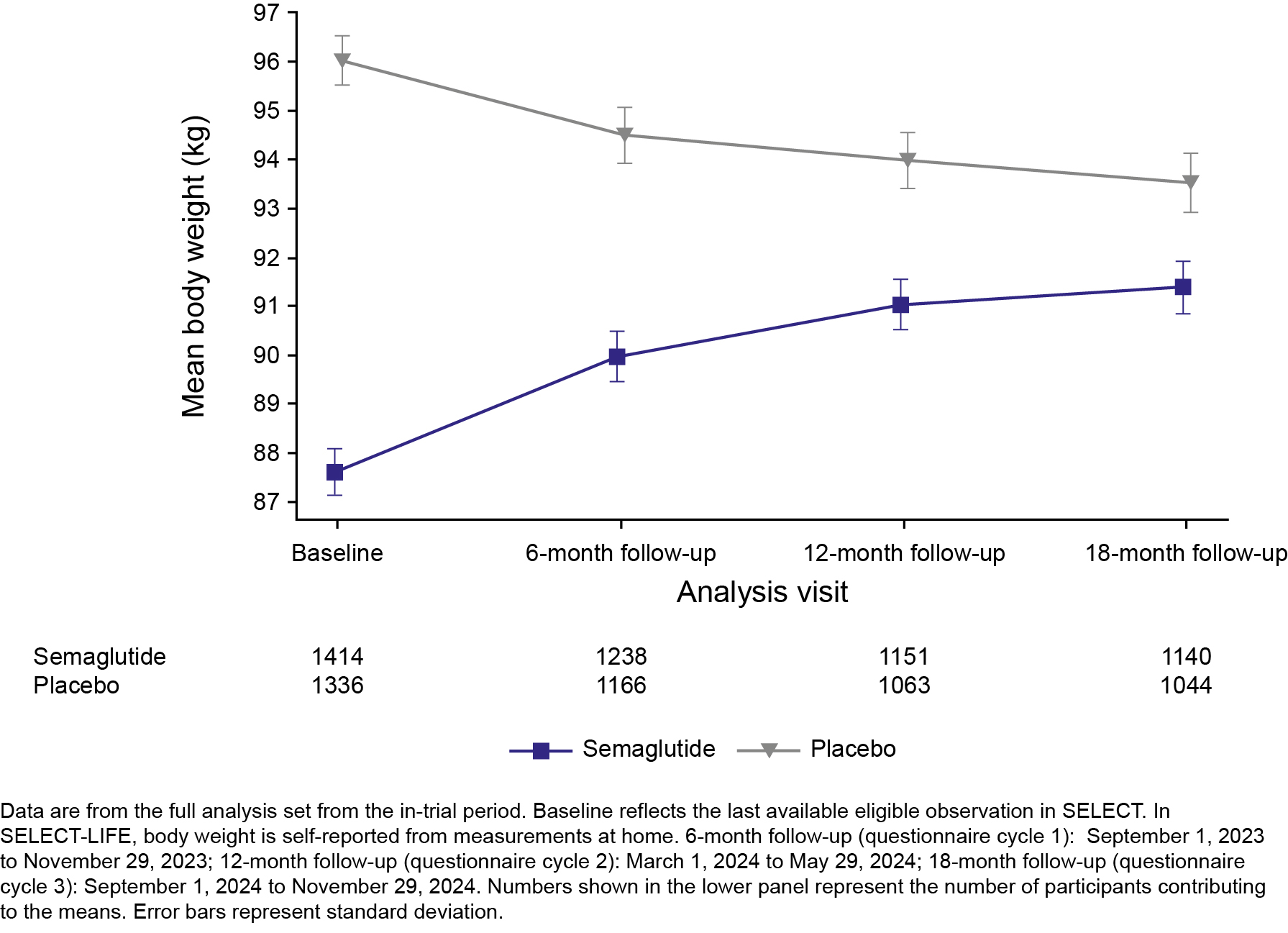
Results: Of 17,604 participants in SELECT, 3435 (19.5%) consented to enroll in SELECT-LIFE; 1760 from the historic SELECT semaglutide arm, and 1675 from the placebo arm. SELECT-LIFE participants were mostly male (76.4%) with a mean age of 64.9 years and body weight of 92.0 kg at study entry. A similar proportion of participants in the historic semaglutide vs placebo arm reported MACE (3.4% vs 3.1%, respectively; Figure 1A) or a new T2D diagnosis (semaglutide: 1.5%; placebo: 1.2%; Figure 1B). Mean (SD) body weight increased from 87.9 kg (17.5) at SELECT-LIFE baseline to 91.4 kg (17.9) in the historic semaglutide arm; and reduced from 96.4 kg (18.6) to 93.5 kg (19.6) in the placebo arm (Figure 2). Numerically fewer participants reported events of cancer, knee replacements, and CPAP device use with historic semaglutide vs placebo (Table 1).
Conclusions: After a median of 16 months post-SELECT completion, there is no additional effect on MACE or progression to T2D after semaglutide study drug discontinuation. However, historic treatment with semaglutide appears to have benefits for knee replacement surgeries and sleep apnea, despite expected partial weight regain.
- Lingvay, Ildiko ( UT Southwestern Medical Center , Dallas , Texas , United States )
- Deanfield, John ( University College London , London , United Kingdom )
- De Los Angeles Quiroga Pelaez, Maria ( Novo Nordisk , Søborg , Denmark )
- Kahn, Steven ( VA Puget Sound Health Care System and University of Washington , Seattle , Washington , United States )
- Lincoff, Abraham ( CLEVELAND CLINIC , Bentleyville , Ohio , United States )
- Plutzky, Jorge ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Ross, Stine ( Novo Nordisk , Søborg , Denmark )
- Weeke, Peter ( Novo Nordisk , Søborg , Denmark )
- Ryan, Donna ( Pennington Biomedical , New Orleans , Louisiana , United States )
Meeting Info:
Session Info:
Optimizing Use of GLP-1 RA for Cardiometabolic Benefit: New Strategies and Applications
Monday, 11/10/2025 , 10:45AM - 12:00PM
Moderated Digital Poster Session
More abstracts on this topic:
Tian Shanshan, Ni Mingke, Wang Hui, Zhu Hai-lei, Wang Ruiwu, Estillore John Paul, Chen Wayne
A short version of HFD/L-NAME mouse model enabling time-effective proof of concept studies to evaluate drugs targeting the cardiometabolic and mild hypertension associated HFpEF phenotype.Assaly Rana, Dubroca Caroline, Waget Aurelie, Perrier Kevin, Sulpice Thierry
More abstracts from these authors:
Verma Subodh, Lincoff Abraham, Emerson Scott, Plutzky Jorge, Kahn Steven, Stensen Signe, Weeke Peter, Rasmussen Soren, Poirier Paul, Lingvay Ildiko
Efficacy and Safety of Semaglutide According to Frailty Status in the SELECT TrialOstrominski John, Sofer Yael, Urina-triana Miguel, Aroda Vanita, Plutzky Jorge, Scirica Benjamin, Hovingh G. Kees, Jeppesen Ole Kleist, Lincoff Abraham, Lingvay Ildiko, Morville Thomas Hoffmann, De Los Angeles Quiroga Pelaez Maria