Final ID: Sa3069
Efficacy and Safety of Semaglutide According to Frailty Status in the SELECT Trial
Background: Frailty is common in people with cardiovascular disease (CVD) and may modify the risks and benefits of therapeutic interventions.
Research Questions: To evaluate the efficacy and safety of once-weekly semaglutide 2.4 mg vs placebo in addition to standard of care in SELECT (Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity) trial participants with BMI ≥27 kg/m2 and CVD but without diabetes, according to baseline frailty status.
Methods: A 31-item frailty index (FI) using medical history, vital signs, laboratory data, and health status measures was constructed using the Rockwood cumulative deficit approach. Frailty status was defined post hoc according to established FI categories at baseline: ≤0.210 (not frail), 0.211–0.310 (more frail), and ≥0.311 (most frail). Treatment effects of semaglutide vs placebo on the primary composite outcome (cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke), key secondary outcomes, and safety events were examined according to baseline FI category.
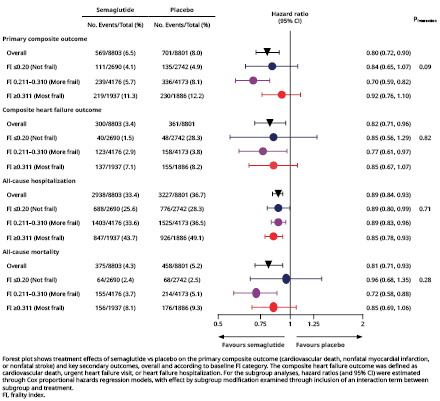
Results: Of 17,604 participants, 5432 (30.9%) had a FI ≤0.210, 8349 (47.4%) had a FI 0.211–0.310, and 3823 (21.7%) had a FI ≥0.311. The incidence of the primary composite outcome increased with higher baseline FI (Figure 1). Benefits of semaglutide vs placebo on the primary composite outcome were consistent across the FI categories (HR 0.84 [95% CI 0.65, 1.07] if FI <0.210; HR 0.70 [95% CI 0.59, 0.82] if FI 0.211–0.310; HR 0.92 [95% CI 0.76, 1.10] if FI ≥0.311; pinteraction=0.09) (Figure 2). Similarly, semaglutide reduced the composite heart failure outcome (pinteraction=0.82), all-cause hospitalization (pinteraction=0.71), and all-cause mortality (pinteraction=0.28) regardless of FI category (Figure 2). Baseline FI category did not appear to modify semaglutide-mediated reductions in body weight (pinteraction=0.08) or improvements in EQ-5D-VAS scores (pinteraction=0.45) by 104 weeks. Relative risks of adverse events leading to permanent study drug discontinuation with semaglutide vs placebo were not increased with higher FI (≤0.210, 15.5% vs 5.5%; 0.211–0.310, 16.8% vs 8.3%; ≥0.311, 17.6% vs 11.7%).
Conclusion: Frailty was common in SELECT, and semaglutide demonstrated beneficial effects on clinical outcomes and health-related quality of life irrespective of baseline frailty status. These findings provide important reassurance around the risk–benefit balance of semaglutide in persons with CVD and overweight or obesity.
- Ostrominski, John ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Sofer, Yael ( Institute of Endocrinology, Diabetes, Metabolism and Hypertension, Tel Aviv-Sourasky Medical Center , Tel Aviv , Israel )
- Urina-triana, Miguel ( Simón Bolívar University , Barranquilla , Colombia )
- Aroda, Vanita ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Plutzky, Jorge ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Scirica, Benjamin ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Hovingh, G. Kees ( Novo Nordisk A/S , Søborg , Denmark )
- Jeppesen, Ole Kleist ( Novo Nordisk A/S , Søborg , Denmark )
- Lincoff, Abraham ( Cleveland Clinic and Cleveland Clinic Lerner College of Medicine of Case Western Reserve University , Cleveland , Ohio , United States )
- Lingvay, Ildiko ( University of Texas Southwestern Medical Center , Dallas , Texas , United States )
- Morville, Thomas Hoffmann ( Novo Nordisk A/S , Søborg , Denmark )
- De Los Angeles Quiroga Pelaez, Maria ( Novo Nordisk A/S , Søborg , Denmark )
Meeting Info:
Session Info:
Metabolic & Lipid-Focused Therapies for CAD
Saturday, 11/08/2025 , 02:30PM - 03:30PM
Abstract Poster Board Session
More abstracts on this topic:
Tian Shanshan, Ni Mingke, Wang Hui, Zhu Hai-lei, Wang Ruiwu, Estillore John Paul, Chen Wayne
A multifaceted family intervention for blood pressure management in rural China: an open label, parallel group, cluster randomized trial (Healthy Family Program)Jiang Chao, Dong Jianzeng, Cai Jun, Anderson Craig, Du Xin, Tang Yangyang, Han Rong, Song Yanna, Wang Chi, Lin Xiaolei, Yi Yang, Rodgers Anthony, Ma Changsheng
More abstracts from these authors:
Lingvay Ildiko, Deanfield John, De Los Angeles Quiroga Pelaez Maria, Kahn Steven, Lincoff Abraham, Plutzky Jorge, Ross Stine, Weeke Peter, Ryan Donna
Semaglutide Improves Cardiovascular Outcomes in Patients with a History of Coronary Artery Bypass Surgery and Overweight or Obesity: The SELECT TrialVerma Subodh, Lincoff Abraham, Emerson Scott, Plutzky Jorge, Kahn Steven, Stensen Signe, Weeke Peter, Rasmussen Soren, Poirier Paul, Lingvay Ildiko