Final ID: 4171808
Semaglutide Improves Cardiovascular Outcomes in Patients with a History of Coronary Artery Bypass Surgery and Overweight or Obesity: The SELECT Trial
Abstract Body (Do not enter title and authors here): Introduction/Background: The SELECT trial demonstrated that subcutaneous semaglutide 2.4 mg once weekly led to a 20% relative risk reduction vs placebo in major adverse cardiovascular (CV) events (MACE) in people with overweight/obesity and CV disease (CVD), but without a history of diabetes. Whether patients with prior coronary artery bypass graft (CABG) benefit from semaglutide remains unclear.
Research Questions/Hypothesis: This prespecified analysis of SELECT assessed differences in baseline characteristics and CV efficacy of semaglutide vs placebo in patients with a history of CABG.
Methods/Approach: SELECT was a multicenter, double-blind, randomized, placebo-controlled, event-driven trial. Participants aged ≥45 years with CVD and a body mass index ≥27 kg/m2, without diabetes were randomized 1:1 to semaglutide 2.4 mg or placebo. The primary CV endpoint was time to first MACE, analyzed with Cox proportional hazards model with treatment as a fixed factor, by CABG history.
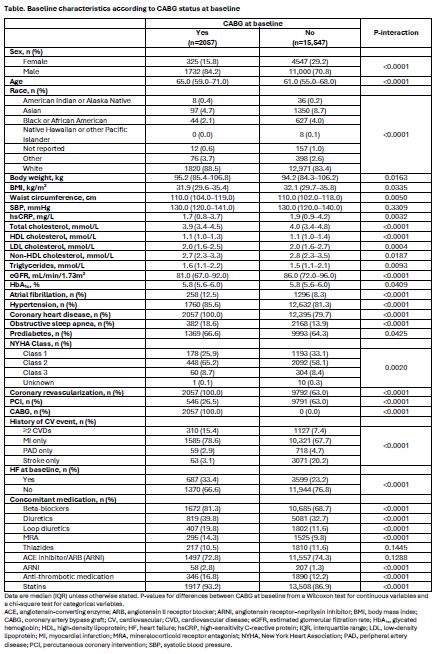
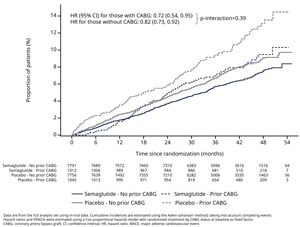
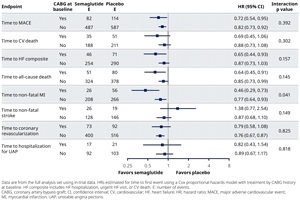
Results/Data: Of 17,604 patients, those with prior CABG (2057 [12%]) were older, more likely male (84%), had higher prevalence of atrial fibrillation, hypertension, heart failure, obstructive sleep apnea, and coronary heart disease, and were more likely to be treated with medications including beta-blockers, diuretics, statins, and anti-thrombotics than those with no prior CABG (Table). Overall, patients with prior CABG were at higher risk of CV events vs those without CABG as seen with placebo (incidence rate of MACE 3.4 vs. 2.3%; CV death 1.5 vs. 0.8%; fatal or non-fatal myocardial infarction [MI] 1.8 vs. 1.1%; extended MACE 5.1 vs. 3.7 % and all-cause death 2.3 vs. 1.5%; respectively). Semaglutide led to consistent reductions in MACE outcomes regardless of CABG history vs placebo (Figure 1). For the primary endpoint, the HR (95% CI) for those with CABG was 0.72 (0.54, 0.95) vs 0.82 (0.73, 0.92) for those without CABG (p-interaction=0.39), with absolute risk differences at week 156 of 2.3% (−0.01%, 4.8%) and 1.0% (0.2%, 1.8%), respectively. The efficacy of semaglutide was generally consistent for all key secondary and confirmatory endpoints evaluated (Figure 2). In patients with CABG at baseline, a lower proportion had serious adverse events in the semaglutide treatment group (38%) vs placebo (44%).
Conclusions: In SELECT, semaglutide consistently reduced CV outcomes regardless of CABG history with greater absolute risk reduction in those with CABG history.
Research Questions/Hypothesis: This prespecified analysis of SELECT assessed differences in baseline characteristics and CV efficacy of semaglutide vs placebo in patients with a history of CABG.
Methods/Approach: SELECT was a multicenter, double-blind, randomized, placebo-controlled, event-driven trial. Participants aged ≥45 years with CVD and a body mass index ≥27 kg/m2, without diabetes were randomized 1:1 to semaglutide 2.4 mg or placebo. The primary CV endpoint was time to first MACE, analyzed with Cox proportional hazards model with treatment as a fixed factor, by CABG history.
Results/Data: Of 17,604 patients, those with prior CABG (2057 [12%]) were older, more likely male (84%), had higher prevalence of atrial fibrillation, hypertension, heart failure, obstructive sleep apnea, and coronary heart disease, and were more likely to be treated with medications including beta-blockers, diuretics, statins, and anti-thrombotics than those with no prior CABG (Table). Overall, patients with prior CABG were at higher risk of CV events vs those without CABG as seen with placebo (incidence rate of MACE 3.4 vs. 2.3%; CV death 1.5 vs. 0.8%; fatal or non-fatal myocardial infarction [MI] 1.8 vs. 1.1%; extended MACE 5.1 vs. 3.7 % and all-cause death 2.3 vs. 1.5%; respectively). Semaglutide led to consistent reductions in MACE outcomes regardless of CABG history vs placebo (Figure 1). For the primary endpoint, the HR (95% CI) for those with CABG was 0.72 (0.54, 0.95) vs 0.82 (0.73, 0.92) for those without CABG (p-interaction=0.39), with absolute risk differences at week 156 of 2.3% (−0.01%, 4.8%) and 1.0% (0.2%, 1.8%), respectively. The efficacy of semaglutide was generally consistent for all key secondary and confirmatory endpoints evaluated (Figure 2). In patients with CABG at baseline, a lower proportion had serious adverse events in the semaglutide treatment group (38%) vs placebo (44%).
Conclusions: In SELECT, semaglutide consistently reduced CV outcomes regardless of CABG history with greater absolute risk reduction in those with CABG history.
More abstracts on this topic:
A Focus for Improvement - Factors for Lab Adherence in a Pediatric Preventive Cardiology Program
Holsinger Hunter, Porterfield Ronna, Taylor Makenna, Dresbach Bethany, Seipel Brittany, Igwe Chukwuemeka, Alvarado Chance, Tran Andrew
Association of Pre-operative Neutrophil to Lymphocyte Ratio (NLR) and Post-operative AKI in Patients Undergoing CABG: A Meta-AnalysisPatel Bhavin, Lapsiwala Boney, Jariwala Prayag, Suresh Aditya, Vanani Samir, Patel Shaurya, Rajani Aayushi, Desai Rupak