Final ID: 4368742
Long-term Impact of Aficamten on Patient-Reported Outcome Measures in Obstructive Hypertrophic Cardiomyopathy: Results From FOREST-HCM
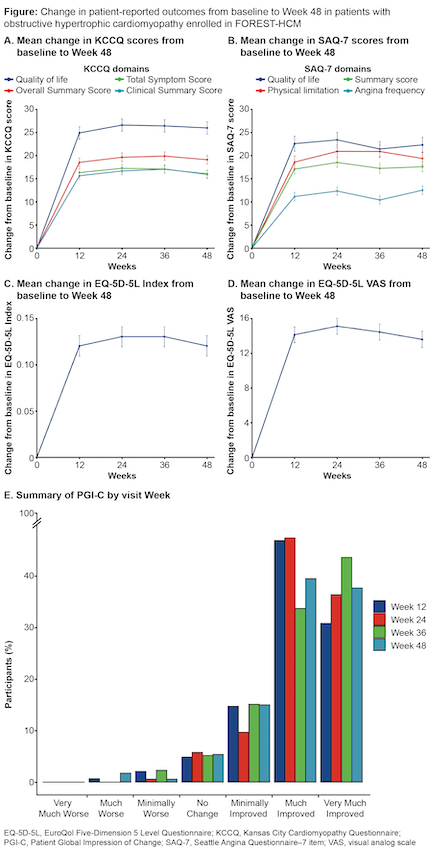
Methods: Patients completing an aficamten parent study were offered participation in FOREST-HCM. Patients completing ≥48 weeks in FOREST-HCM as of August 31, 2024 were included. The Kansas City Cardiomyopathy Questionnaire (KCCQ), Seattle Angina Questionnaire 7-item (SAQ-7), and EuroQol Five-Dimensional Questionnaire (EQ-5D-5L) index and visual analog scale (VAS) were administered at baseline and at Weeks 12, 24, 36, and 48. The patient global impression of change (PGI-C) survey was administered at Weeks 12, 24, 36, and 48. Mixed models for repeated measures were used to analyze changes in mean KCCQ summary scores (including the Clinical Summary Score [CSS] and Overall Summary Score [OSS]), SAQ-7 summary scores, and EQ-5D-5L index and VAS scores from baseline to 48 weeks. Associations among PROs and between PROs and clinical measures of HCM severity were evaluated using Spearman correlation coefficients.
Results: As of August 2024, 182 patients (mean age 60.2 [SD ±13.1] years, 56% were male) had reached 48 weeks of follow-up. Significant improvements in all PROs were observed by Week 12 and sustained through Week 48 (Figure). Least-squares mean differences (95% CI) between the KCCQ-CSS and -OSS from baseline to 48 weeks were 16.1 (14.4–17.7) and 19.1 (17.3–21.0), respectively (P<0.0001), with the largest gains in the quality-of-life (QoL) domain (26.0 [23.3–28.6]; P<0.0001). The SAQ-7 summary score increased by 17.7 (15.5–19.8; P<0.0001), with a 22.4-point gain (19.1–25.6; P<0.0001) in the QoL domain. EQ-5D-5L index and VAS scores improved by 0.12 (0.10–0.14) and 13.4 (11.6–15.2), respectively (P<0.0001). At 48 weeks, 77% of patients reported feeling “Much Improved” or “Very Much Improved” on the PGI-C. Improvements in PROs were significantly correlated with important clinical measures of oHCM severity (Table).
Conclusions: Aficamten led to significant and sustained improvements in PROs in patients with oHCM. The improvement was seen across all symptom domains, particularly QoL, and correlated with important clinical measures of disease severity.
- Weiner, Shepard ( Columbia University Irving Medical Center , New York , New York , United States )
- Oreziak, Artur ( National Institute of Cardiology , Warsaw , Poland )
- Saberi, Sara ( University of Michigan , Ann Arbor , Michigan , United States )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Spertus, John ( Saint Luke's Mid America Heart Institute , Kansas City , Missouri , United States )
- Tower Rader, Albree ( Massachusetts General Hospital , Boston , Massachusetts , United States )
- Butzner, Michael ( Cytokinetics Inc. , South San Francisco , California , United States )
- Heitner, Stephen ( Cytokinetics Inc. , South San Francisco , California , United States )
- Jacoby, Daniel ( Cytokinetics Inc. , South San Francisco , California , United States )
- Kupfer, Stuart ( Cytokinetics Inc. , South San Francisco , California , United States )
- Liu, Xueli ( Cytokinetics Inc. , South San Francisco , California , United States )
- Liang, Lusha ( NYP , New York , New York , United States )
- Malik, Fady ( Cytokinetics Inc. , South San Francisco , California , United States )
- Melloni, Chiara ( Cytokinetics Inc. , South San Francisco , California , United States )
- Simkins, Tyrell ( Cytokinetics Inc. , South San Francisco , California , United States )
- Wei, Jenny ( Cytokinetics Inc. , South San Francisco , California , United States )
- Nassif, Michael ( University of Missouri Kansas City Healthcare Institute for Innovations in Quality and Saint Luke’s Mid America Heart Institute , Kansas City , Missouri , United States )
- Owens, Anjali ( University of Pennsylvania , Wallingford , Pennsylvania , United States )
- Masri, Ahmad ( OHSU , Portland , Oregon , United States )
- Abraham, Theodore ( Univ of California at San Francisco , San Francisco , California , United States )
- Barriales-villa, Roberto ( Complexo Hospitalario Universitario , A Coruna , Spain )
- Cooper, Robert ( Liverpool Heart and Chest Hospital , Liverpool , United Kingdom )
- Elliott, Perry ( University College London , London , United Kingdom )
- De Feria, Alejandro ( University of Pennsylvania , Philadelphia , Pennsylvania , United States )
- Maron, Martin ( Lahey Hospital and Medical Center , Burlington , Massachusetts , United States )
Meeting Info:
Session Info:
Hypertrophic Cardiomyopathy Medical Society Oral Abstracts
Friday, 11/07/2025 , 02:30PM - 03:45PM
Abstract Oral Session
More abstracts on this topic:
Lee Matthew, Malik Fady, Kupfer Stuart, Wohltman Amy, Coats Caroline, Abraham Theodore, Claggett Brian, Maron Martin, Miao Zi, Meder Benjamin, Olivotto Iacopo, Heitner Stephen, Jacoby Daniel
AI-Driven Electrocardiographic Detection and Subtyping of Hypertrophic Cardiomyopathy: A Deep Learning Approach Using 12-Lead ECGsSoh Moon Seung, Yu Taehyung, Na Yeongyeon, Joo Sunghoon, Shin Joon-han
More abstracts from these authors:
Masri Ahmad, Naidu Srihari, Nassif Michael, Olivotto Iacopo, Oreziak Artur, Owens Anjali, Wever-pinzon Omar, Tower Rader Albree, Heitner Stephen, Kupfer Stuart, Malik Fady, Choudhury Lubna, Melloni Chiara, Meng Lixin, Wei Jenny, Saberi Sara, Garcia-pavia Pablo, Abraham Theodore, Barriales-villa Roberto, Bilen Ozlem, Elliott Perry, Hagege Albert, Nagueh Sherif
Chronic Aficamten Treatment Results in Sustained Favorable Cardiac Remodeling in Patients with Symptomatic Obstructive Hypertrophic Cardiomyopathy: Insights From the FOREST-HCM TrialHegde Sheila, Oreziak Artur, Owens Anjali, Tower Rader Albree, Heitner Stephen, Jacoby Daniel, Kupfer Stuart, Liu Xueli, Malik Fady, Melloni Chiara, Simkins Tyrell, Pabon Maria, Wei Jenny, Solomon Scott, Saberi Sara, Masri Ahmad, Nassif Michael, Abraham Theodore, Barriales-villa Roberto, Cooper Robert, Elliott Perry, Maron Martin