Final ID: MP1352
Nutrition in the Cardiac Intensive Care Unit: Insights from a Survey of the Critical Care Cardiology Trials Network
Abstract Body (Do not enter title and authors here): Background: Nutrition is a vital component of recovery for critically ill cardiac patients. However, there is a substantial lack of evidence, consensus, and guidelines regarding optimal nutrition strategies for critically ill cardiac patients, particular those presenting with cardiogenic shock.
Methods: The Critical Care Cardiology Trials Network (CCCTN) is an international multicenter research network consisting of advanced cardiac intensive care units (CICUs). A nutrition-based survey was distributed to CCCTN sites in 2024-2025.
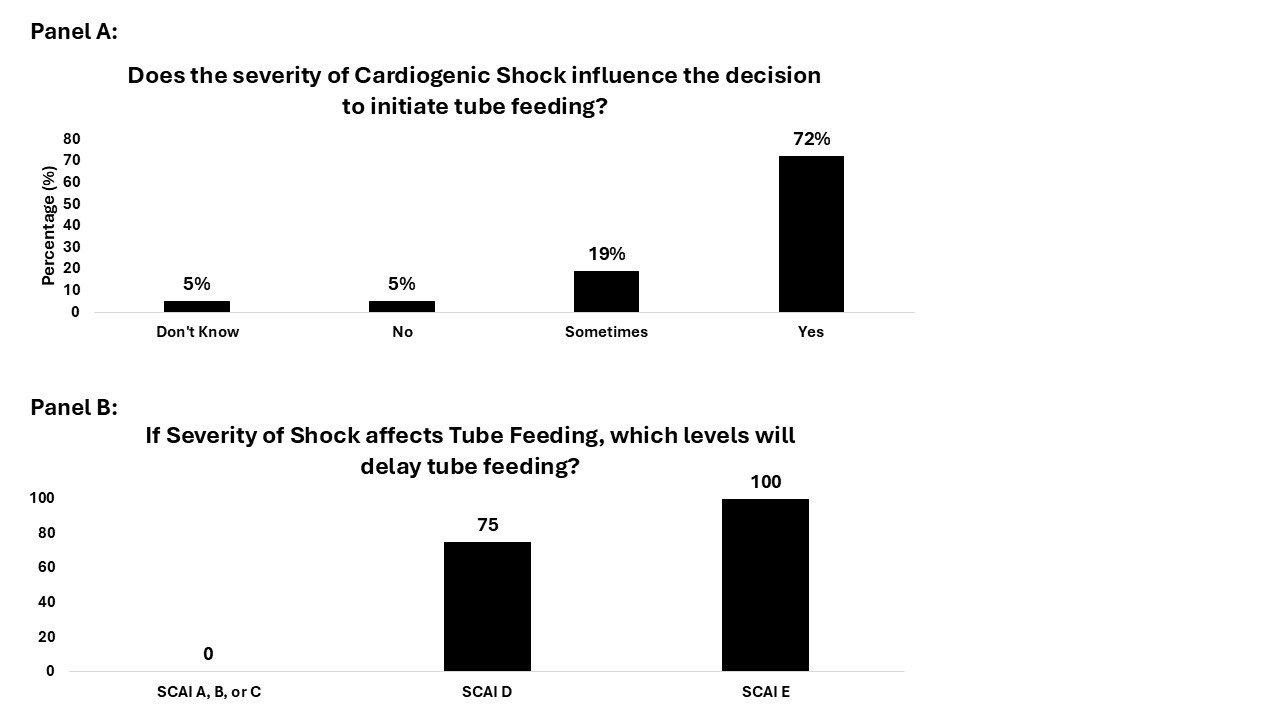
Results: A total of 43 sites responded to the survey. ASPEN nutrition guidelines are most commonly used to inform local protocols and nutrition practice (86%) however a large portion of sites also follow individual institutional protocols (65%). For malnourished patients, 49% of sites initiate nutrition 24-48 hours after admission, compared with 44% for non-malnourished patients. Most sites initiate enteral nutrition at a low rate and advance to goal hourly rates, reported at 88% of sites for malnourished and 93% for non-malnourished patients. Severity of cardiogenic shock influences the decision to initiate tube feeding at 91% of sites, with SCAI D and E very frequently delaying tube feeding. Elevated protein targets (1.5–2.0 g/kg/day) are prescribed at 47% of sites for malnourished patients and 12% of sites for non-malnourished patients. Supplemental parenteral nutrition (SPN) is sparsely used for malnourished patients, or when reaching EN is challenging with 7% of sites starting SPN at baseline and 49% starting SPN if energy and protein targets cannot be achieved. The PN formula used varies with 30% of sites using mixed oil intravenous lipid emulsion (ILE) without fish oil, 74% using Mixed oil ILE with fish oil, and 28% using 100% soybean oil ILE .
Conclusions: There is significant variability in nutrition practices across contemporary North American CICUs. The severity of cardiogenic shock (SCAI D/E) prompted sites to delay initiation of nutrition. Despite recent data suggesting harm from high dose protein supplementation and benefit from a mixed oil/fish oil ILE for critically ill patients, > 45% of sites employed high protein targets and over a quarter of sites employed 100% soybean oil ILE.
Methods: The Critical Care Cardiology Trials Network (CCCTN) is an international multicenter research network consisting of advanced cardiac intensive care units (CICUs). A nutrition-based survey was distributed to CCCTN sites in 2024-2025.
Results: A total of 43 sites responded to the survey. ASPEN nutrition guidelines are most commonly used to inform local protocols and nutrition practice (86%) however a large portion of sites also follow individual institutional protocols (65%). For malnourished patients, 49% of sites initiate nutrition 24-48 hours after admission, compared with 44% for non-malnourished patients. Most sites initiate enteral nutrition at a low rate and advance to goal hourly rates, reported at 88% of sites for malnourished and 93% for non-malnourished patients. Severity of cardiogenic shock influences the decision to initiate tube feeding at 91% of sites, with SCAI D and E very frequently delaying tube feeding. Elevated protein targets (1.5–2.0 g/kg/day) are prescribed at 47% of sites for malnourished patients and 12% of sites for non-malnourished patients. Supplemental parenteral nutrition (SPN) is sparsely used for malnourished patients, or when reaching EN is challenging with 7% of sites starting SPN at baseline and 49% starting SPN if energy and protein targets cannot be achieved. The PN formula used varies with 30% of sites using mixed oil intravenous lipid emulsion (ILE) without fish oil, 74% using Mixed oil ILE with fish oil, and 28% using 100% soybean oil ILE .
Conclusions: There is significant variability in nutrition practices across contemporary North American CICUs. The severity of cardiogenic shock (SCAI D/E) prompted sites to delay initiation of nutrition. Despite recent data suggesting harm from high dose protein supplementation and benefit from a mixed oil/fish oil ILE for critically ill patients, > 45% of sites employed high protein targets and over a quarter of sites employed 100% soybean oil ILE.
More abstracts on this topic:
Admission Acid-Base Status and Mortality in Cardiac Intensive Care Unit Patients
Canova Tyler, Lipps Kirsten, Hillerson Dustin, Kashani Kianoush, Dahiya Garima, Jentzer Jacob
Exploring Facilitators and Barriers for Personalized Dietary Incentives among Online Shoppers at Cardiovascular Risk and Key Informants to Inform an Automated Shopping PlatformVadiveloo Maya, Elenio Emily, Tovar Alison, Thorndike Anne