Final ID: 4367913
The Highly Electronegative HDL Subfraction, H5, Promotes Cell Dysfunction via Ferroptosis
Abstract Body (Do not enter title and authors here): Background:
Ferroptosis is a regulated, iron-dependent form of cell death characterized by the accumulation of lipid peroxides and oxidative stress. The process is closely linked to disruptions in iron and lipid metabolism and is increasingly recognized as a contributor to cancer, neurodegenerative disorders, and cardiovascular diseases (CVD). HDL is traditionally viewed as atheroprotective; however, subfractional heterogeneity complicates this notion. H5, the most electronegative HDL subfraction, is enriched in apoC-III and triglycerides and has been shown to exert pro-inflammatory effects, disrupt redox balance, and impair endothelial function. Yet, its direct cytotoxic potential has not been fully elucidated.
Research question:
This study aimed to determine whether H5 induces ferroptosis-mediated cellular dysfunction, extending beyond its pro-inflammatory properties. We investigated the impact of H5 on ferroptosis markers and oxidative stress responses in human umbilical vein endothelial cells (HUVECs) and Caenorhabditis elegans (C. elegans), to assess its potential as a therapeutic target.
Methods:
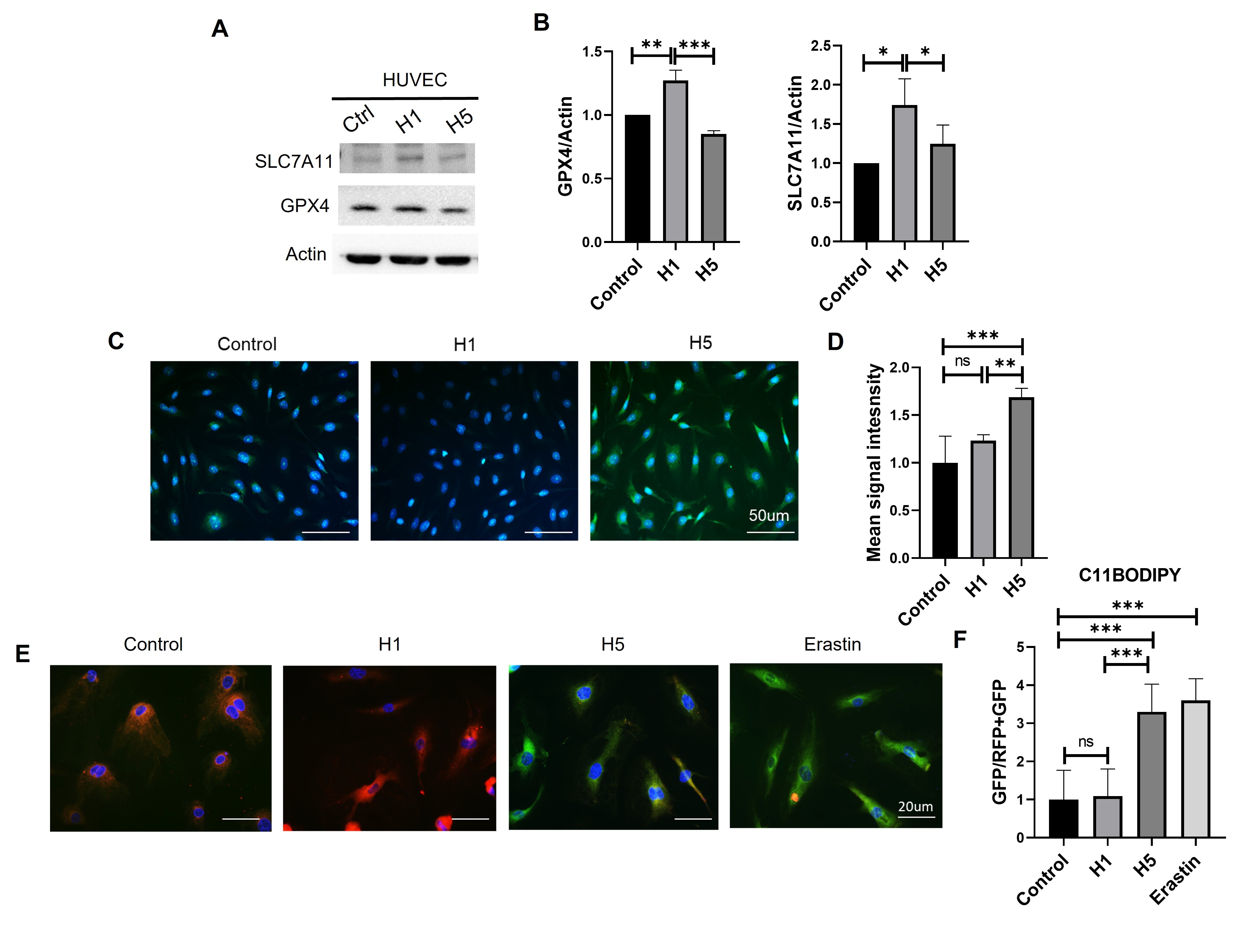
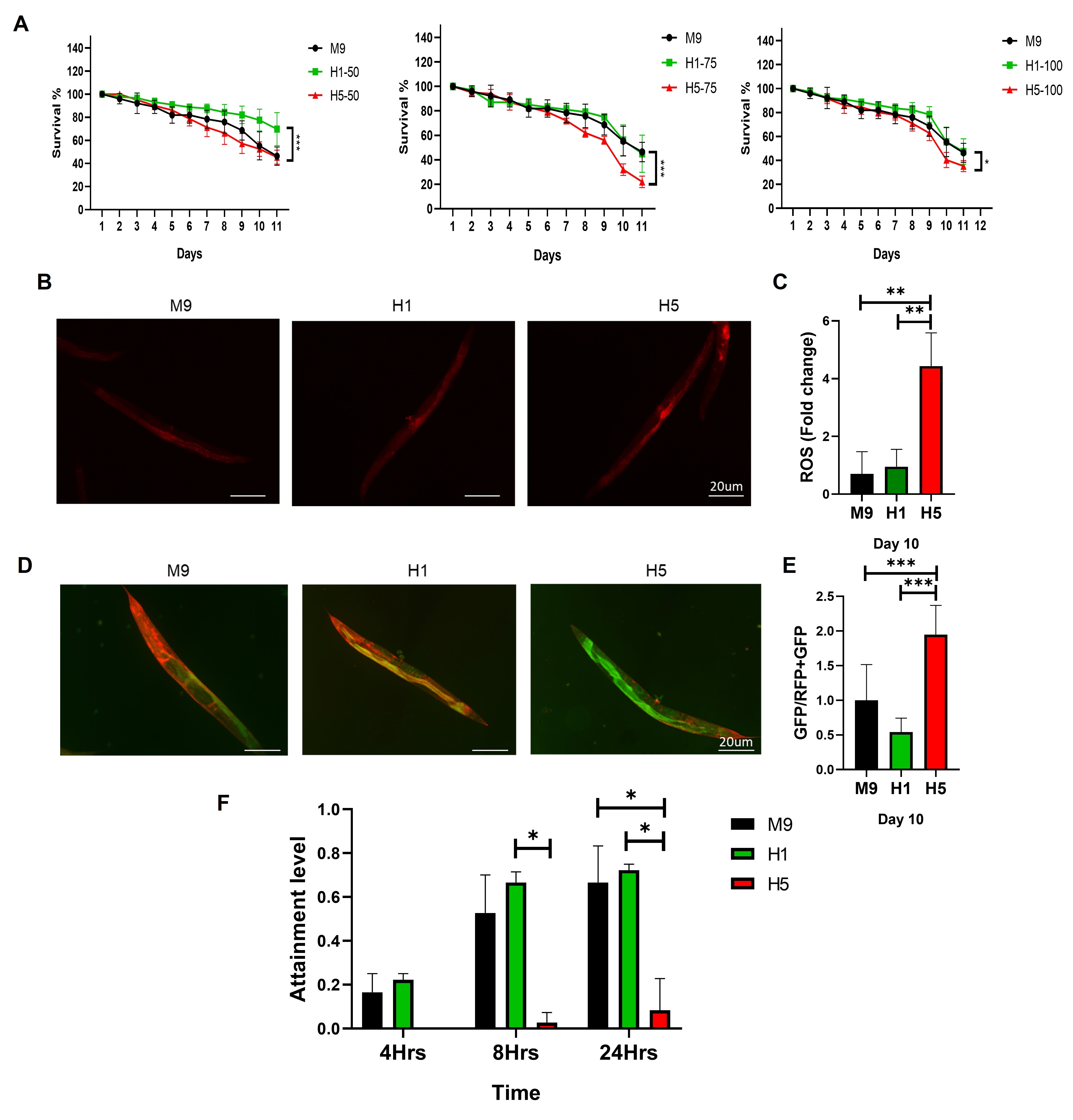
H1 and H5, representing the least and most electronegative HDL subfractions respectively, were chromatographically isolated from pooled human plasma. HUVECs were exposed to H1 or H5 for 24 hours, followed by assessment of key ferroptosis markers including SLC7A11 and GPX4 (via Western blot), total reactive oxygen species (ROS; CellROX immunofluorescence), and lipid peroxidation (C11-BODIPY staining). For in vivo validation, H5 dosage was optimized in C. elegans through survival assays. Treated worms were then evaluated for ROS production, lipid peroxidation, and neuronal function using a foraging behavioral assay.
Results:
H5-treated HUVECs exhibited significant downregulation of SLC7A11 and GPX4, coupled with elevated ROS and lipid peroxidation levels, indicating induction of ferroptosis. In C. elegans, 75 µg/mL H5 significantly impaired survival, increased oxidative stress, and elevated lipid peroxidation. H5 exposure also reduced foraging behavior, suggesting neuronal dysfunction and broader physiological impairment.
Conclusions:
Our findings demonstrate that H5 induces ferroptosis-mediated endothelial and systemic dysfunction in vitro and in vivo. These results reveal a direct cytotoxic role for electronegative HDL and suggest that targeting H5 could represent a novel therapeutic strategy to mitigate CVD progression and its systemic manifestations.
Ferroptosis is a regulated, iron-dependent form of cell death characterized by the accumulation of lipid peroxides and oxidative stress. The process is closely linked to disruptions in iron and lipid metabolism and is increasingly recognized as a contributor to cancer, neurodegenerative disorders, and cardiovascular diseases (CVD). HDL is traditionally viewed as atheroprotective; however, subfractional heterogeneity complicates this notion. H5, the most electronegative HDL subfraction, is enriched in apoC-III and triglycerides and has been shown to exert pro-inflammatory effects, disrupt redox balance, and impair endothelial function. Yet, its direct cytotoxic potential has not been fully elucidated.
Research question:
This study aimed to determine whether H5 induces ferroptosis-mediated cellular dysfunction, extending beyond its pro-inflammatory properties. We investigated the impact of H5 on ferroptosis markers and oxidative stress responses in human umbilical vein endothelial cells (HUVECs) and Caenorhabditis elegans (C. elegans), to assess its potential as a therapeutic target.
Methods:
H1 and H5, representing the least and most electronegative HDL subfractions respectively, were chromatographically isolated from pooled human plasma. HUVECs were exposed to H1 or H5 for 24 hours, followed by assessment of key ferroptosis markers including SLC7A11 and GPX4 (via Western blot), total reactive oxygen species (ROS; CellROX immunofluorescence), and lipid peroxidation (C11-BODIPY staining). For in vivo validation, H5 dosage was optimized in C. elegans through survival assays. Treated worms were then evaluated for ROS production, lipid peroxidation, and neuronal function using a foraging behavioral assay.
Results:
H5-treated HUVECs exhibited significant downregulation of SLC7A11 and GPX4, coupled with elevated ROS and lipid peroxidation levels, indicating induction of ferroptosis. In C. elegans, 75 µg/mL H5 significantly impaired survival, increased oxidative stress, and elevated lipid peroxidation. H5 exposure also reduced foraging behavior, suggesting neuronal dysfunction and broader physiological impairment.
Conclusions:
Our findings demonstrate that H5 induces ferroptosis-mediated endothelial and systemic dysfunction in vitro and in vivo. These results reveal a direct cytotoxic role for electronegative HDL and suggest that targeting H5 could represent a novel therapeutic strategy to mitigate CVD progression and its systemic manifestations.
More abstracts on this topic:
A Novel Cardioprotective Mechanism in Myocardial Reperfusion Injury: Dual Neutrophil Modulation and ROS/HOCl Scavenging by an Atypical Chemokine
Zwissler Leon, Bernhagen Juergen, Cabrera-fuentes Hector Alejandro, Hernandez Resendiz Sauri, Yap En Ping, Schindler Lisa, Zhang Zhishen, Dickerhof Nina, Hampton Mark, Liehn Elisa, Hausenloy Derek
Acetylation of Mitochondrial Cyclophilin D Increases vascular Oxidative Stress, Induces Glycolitic Switch, Promotes Endothelial Dysfunction and HypertensionDikalov Sergey, Sack Michael, Dikalova Anna, Fehrenbach Daniel, Mayorov Vladimir, Panov Alexander, Ao Mingfang, Lantier Louise, Amarnath Venkataraman, Lopez Marcos, Billings Frederic