Final ID: MP1046
In Vitro Investigation of the Pathophysiological Mechanisms Underlying Severe Cardiomyopathic Phenotypes Associated with Noonan Syndrome with Multiple Lentigines
Abstract Body (Do not enter title and authors here): [Background]
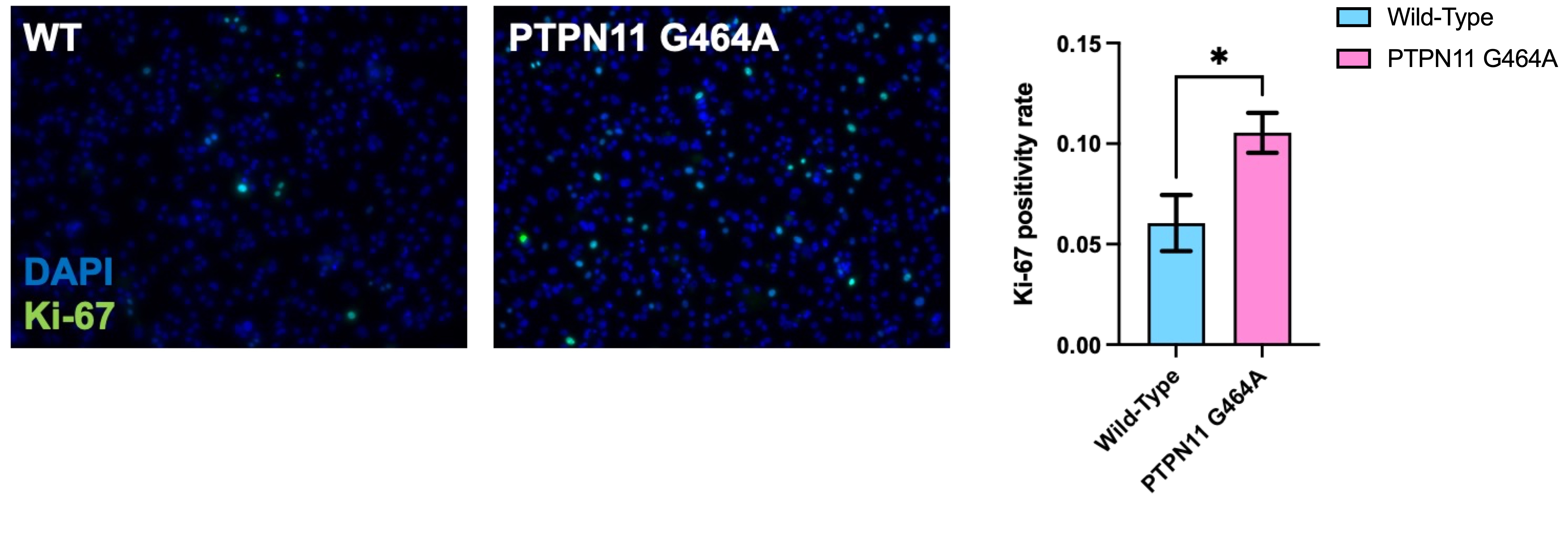
RASopathy-associated cardiomyopathy (RAS-CMP) is thought to involve abnormalities in cardiomyocyte differentiation and maturation. In our previous work, we identified the presence of Ki-67-labeled cardiomyocytes in the hearts of patients with Noonan syndrome with multiple lentigines (NSML) and related forms of RAS-CMP. In this study, we employed a cellular model of NSML-associated cardiomyopathy (NSML-CMP) to investigate the molecular determinants of cardiomyocyte proliferative potential and to elucidate the contribution of these Ki-67-labeled cardiomyocytes to the pronounced myocardial thickening observed in NSML-CMP.
[Methods]
A NSML-causing, PTPN11 G464A pathogenic mutation was introduced to wild-type iPS cells by the CRISPR-Cas technique, and both mutant and wild-type iPS cells were differentiated into cardiomyocytes (iPSCMs) to generate a NSML-CMP model and its isogenic control. Phenotypic analyses were followed by profiling of the underlying aberrancies in mitogenic signaling.
[Results]
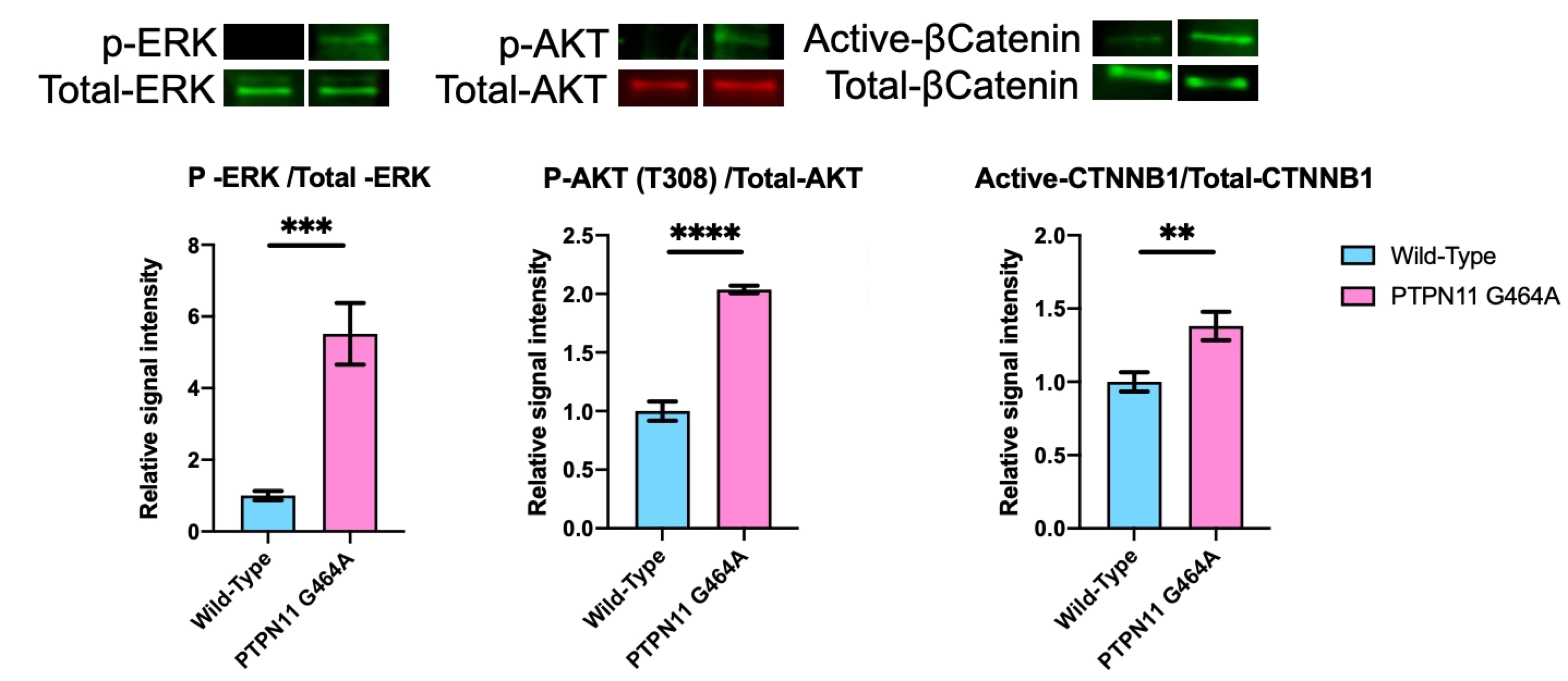
The PTPN11 G464A mutant iPSCMs recapitulated the increased Ki-67-labeling (p<0.01, Fig. 1) and accentuation in downstream Receptor tyrosine kinases(RTK)signaling (p<0.001), seen in cardiomyocytes of NSML-CMP and other related RAS-CMP forms. Pulldown experiments using recombinant PTPN11, as well as its mutant form, identified a candidate partner protein that may serve as a key linker between RTK signaling and other cooperating mitogenic pathways (Fig. 2).
[Discussion]
We have successfully established a cellular model of NSML-CMP that recapitulates key disease features. Aberrant RTK signaling, further amplified by interacting mitogenic networks, appears to drive the enhanced proliferative potential of cardiomyocytes and pronounced myocardial thickening observed in NSML-CMP. Pharmacological intervention and metabolic profiling are currently underway to achieve phenotypic reversal and to uncover novel druggable molecular abnormalities in NSML-CMP beyond mTORC inhibition.
RASopathy-associated cardiomyopathy (RAS-CMP) is thought to involve abnormalities in cardiomyocyte differentiation and maturation. In our previous work, we identified the presence of Ki-67-labeled cardiomyocytes in the hearts of patients with Noonan syndrome with multiple lentigines (NSML) and related forms of RAS-CMP. In this study, we employed a cellular model of NSML-associated cardiomyopathy (NSML-CMP) to investigate the molecular determinants of cardiomyocyte proliferative potential and to elucidate the contribution of these Ki-67-labeled cardiomyocytes to the pronounced myocardial thickening observed in NSML-CMP.
[Methods]
A NSML-causing, PTPN11 G464A pathogenic mutation was introduced to wild-type iPS cells by the CRISPR-Cas technique, and both mutant and wild-type iPS cells were differentiated into cardiomyocytes (iPSCMs) to generate a NSML-CMP model and its isogenic control. Phenotypic analyses were followed by profiling of the underlying aberrancies in mitogenic signaling.
[Results]
The PTPN11 G464A mutant iPSCMs recapitulated the increased Ki-67-labeling (p<0.01, Fig. 1) and accentuation in downstream Receptor tyrosine kinases(RTK)signaling (p<0.001), seen in cardiomyocytes of NSML-CMP and other related RAS-CMP forms. Pulldown experiments using recombinant PTPN11, as well as its mutant form, identified a candidate partner protein that may serve as a key linker between RTK signaling and other cooperating mitogenic pathways (Fig. 2).
[Discussion]
We have successfully established a cellular model of NSML-CMP that recapitulates key disease features. Aberrant RTK signaling, further amplified by interacting mitogenic networks, appears to drive the enhanced proliferative potential of cardiomyocytes and pronounced myocardial thickening observed in NSML-CMP. Pharmacological intervention and metabolic profiling are currently underway to achieve phenotypic reversal and to uncover novel druggable molecular abnormalities in NSML-CMP beyond mTORC inhibition.
More abstracts on this topic:
A Single-Short Partial Reprogramming of the Endothelial Cells Decreases Blood Pressure Via Attenuation of Endothelial-to-Mesenchymal Transition in Hypertensive Mice
Pernomian Laena, Sinclair David, Mccarthy Cam, Tan Wenbin, Wenceslau Camilla, Waigi Emily, Nguyen Vi, Mohammed Ahmed, Januarioda Costa Tiago, Fontes Milene, Kubinack Jason, Aitken Andrew, Biancardi Vinicia
Acute Severe Mitral Regurgitation Due to Flail Posterior Leaflet without Chordal Rupture Following Myosin Inhibitor Treatment of Hypertrophic Obstructive CardiomyopathyPatel Shreyan, Taha Israa, Elmi Daniel, Shirani Jamshid