Final ID: MDP1379
LMNA p.Q353R Mutation Causes Dilated Cardiomyopathy through Impaired Vitamin D Signaling
Abstract Body (Do not enter title and authors here): Introduction: Dilated cardiomyopathy (DCM) is one of the causative diseases of heart failure, caused by genetic mutations. Cases with mutations in the LMNA gene, which encodes lamin A/C protein and constitutes the nuclear lamina, are known to have a poor prognosis. We have previously shown that the DNA damage accumulation and the consequent activation of DDR in cardiomyocytes are the cause of cardiac function impairment. However, the method to reduce DNA damage in the heart has not been elucidated. Therefore, we decided to explore the compounds that could reduce it in the cardiomyocytes.
Methods and Results: We generated iPS cells from DCM patients with a LMNA mutation (p.Q353R) and differentiated them into cardiomyocytes. The mutant cardiomyocytes showed severe deformation of nuclear morphology as well as reduced contractility. In addition, the number of positive foci for γH2AX, a DNA double-strand break marker, was increased in the nuclei of the mutant strain. These results indicate that in vitro disease modeling using iPS cell-derived cardiomyocytes was successfully achieved.
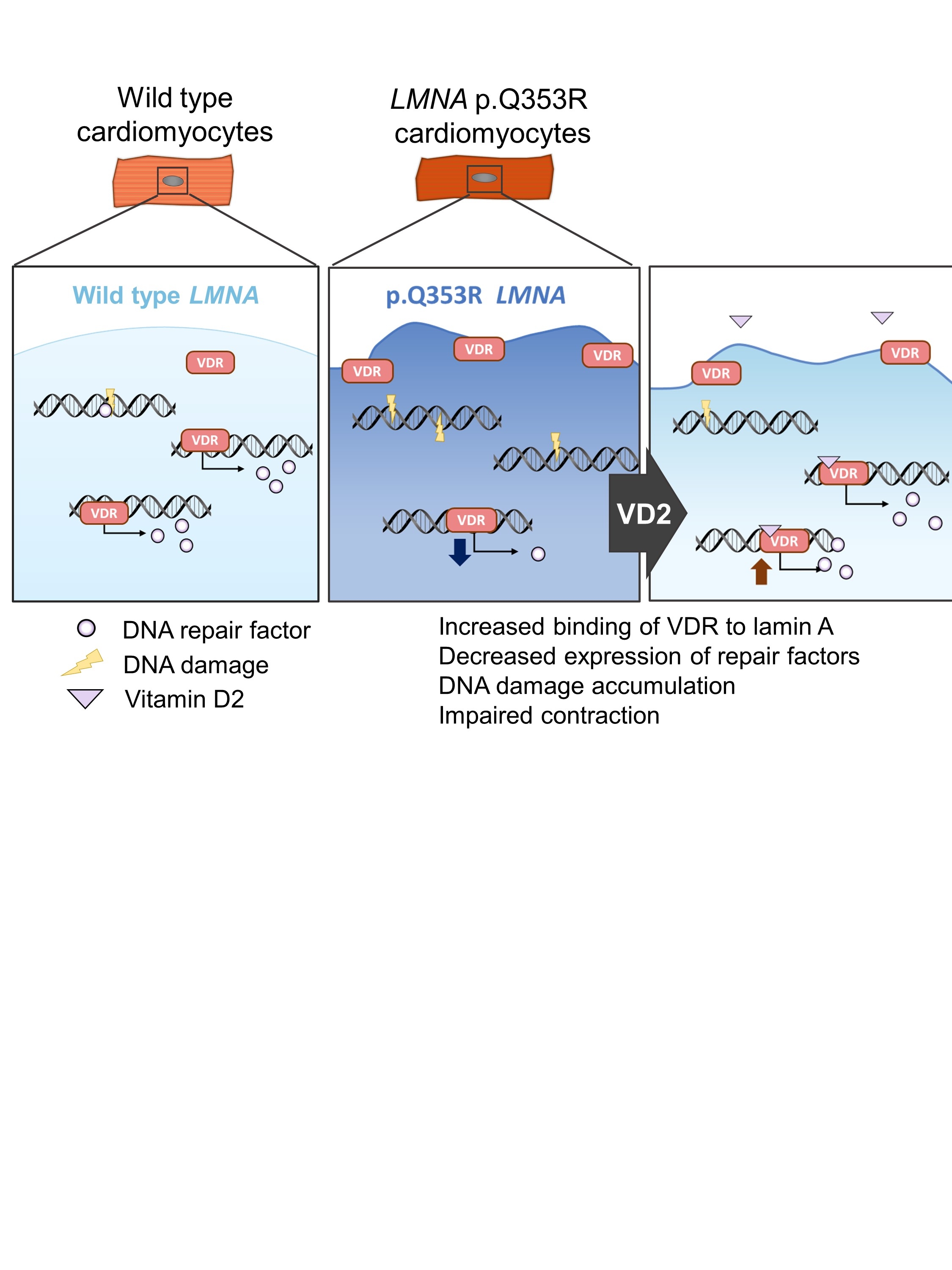
We administered a library of 175 compounds to the mutant cardiomyocytes to search for compounds that reduce the positive foci of γH2AX. We found that vitamin D2 (VD2) significantly reduced DNA damage in the mutant strains. RNA-seq analysis revealed that VD2 restored the expression of DNA repair enzymes that were downregulated in the mutant strains.
We then screened binding molecules of the mutant LMNA p.Q353R protein and wild-type LMNA protein using a protein array. The screening results showed that the binding to the vitamin D receptor (VDR) was significantly enhanced in the mutant protein compared to the wild-type protein. In the mutant cardiomyocytes, VDR was localized to the periphery of the nuclear membrane, and reporter assays showed decreased transcriptional activity of VDR.
Finally, we examined the effects of VD2 on DNA damage accumulation and cardiac function in vivo. VD2 analog reduced DNA damage accumulation and improved cardiac function in two mouse models: a pressure-loaded heart failure model and a Lmna nonsense mutant cardiomyopathy model.
Conclusion: Using patient-derived iPS cells, we could recapitulate various pathological processes in diseased cardiomyocytes, as well as identify therapeutic candidates through compound screening. One of the existing drugs, VD2, was a hit in this screening, and clinical trials for drug repositioning are expected.
Methods and Results: We generated iPS cells from DCM patients with a LMNA mutation (p.Q353R) and differentiated them into cardiomyocytes. The mutant cardiomyocytes showed severe deformation of nuclear morphology as well as reduced contractility. In addition, the number of positive foci for γH2AX, a DNA double-strand break marker, was increased in the nuclei of the mutant strain. These results indicate that in vitro disease modeling using iPS cell-derived cardiomyocytes was successfully achieved.
We administered a library of 175 compounds to the mutant cardiomyocytes to search for compounds that reduce the positive foci of γH2AX. We found that vitamin D2 (VD2) significantly reduced DNA damage in the mutant strains. RNA-seq analysis revealed that VD2 restored the expression of DNA repair enzymes that were downregulated in the mutant strains.
We then screened binding molecules of the mutant LMNA p.Q353R protein and wild-type LMNA protein using a protein array. The screening results showed that the binding to the vitamin D receptor (VDR) was significantly enhanced in the mutant protein compared to the wild-type protein. In the mutant cardiomyocytes, VDR was localized to the periphery of the nuclear membrane, and reporter assays showed decreased transcriptional activity of VDR.
Finally, we examined the effects of VD2 on DNA damage accumulation and cardiac function in vivo. VD2 analog reduced DNA damage accumulation and improved cardiac function in two mouse models: a pressure-loaded heart failure model and a Lmna nonsense mutant cardiomyopathy model.
Conclusion: Using patient-derived iPS cells, we could recapitulate various pathological processes in diseased cardiomyocytes, as well as identify therapeutic candidates through compound screening. One of the existing drugs, VD2, was a hit in this screening, and clinical trials for drug repositioning are expected.
More abstracts on this topic:
A Case of Hypertrophic Cardimyopathy: Digenic Variants of Uncertain Significance Mutations in MHY7 and RYR2 Genes
Durukan Selina, Uzunoglu Ekin, Farahmandsadr Maryam, Soffer Daniel
9-Year Longitudinal Assessment of the 12-lead Electrocardiogram of Volunteer FirefightersBae Alexander, Dzikowicz Dillon, Lai Chi-ju, Brunner Wendy, Krupa Nicole, Carey Mary, Tam Wai Cheong, Yu Yichen