Final ID: 4367427
A Multimodal Artificial Intelligence Signature of Advanced Cardiac and Vascular Aging Defines Elevated Risk of Cardiovascular Disease
Abstract Body (Do not enter title and authors here): BACKGROUND
Age is the strongest factor in current cardiovascular risk estimation tools, yet there is significant heterogeneity in chronological age as a proxy for biological decline. Artificial intelligence (AI) models can infer biological age from routinely collected, non-invasive biomedical data. Here, we introduce a novel cross-modal AI-aging framework that leverages cardiac (ECG) and microvascular (retinal fundus images) aging phenotypes and its association with cardiovascular outcomes.
METHODS
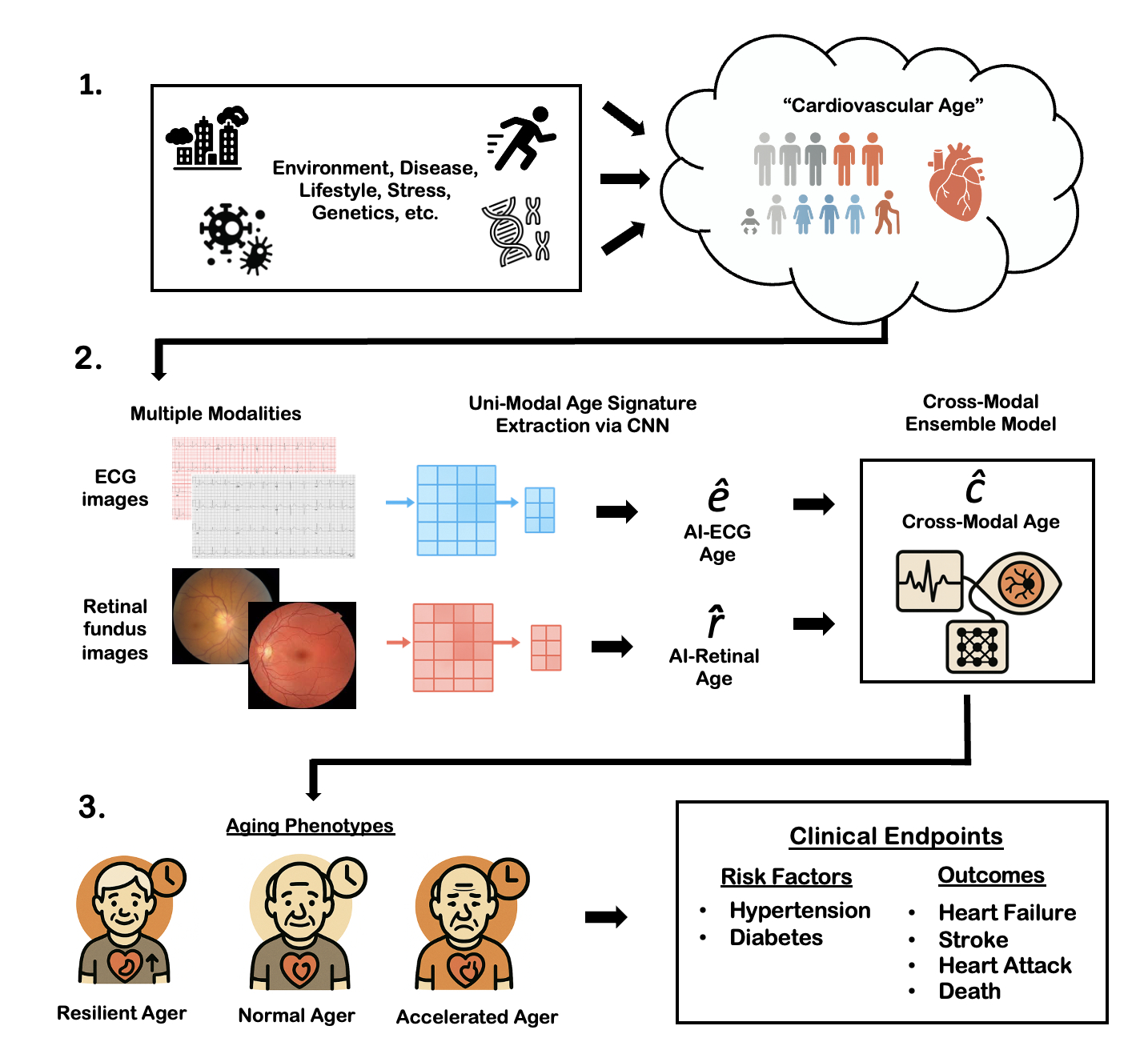
For model development, we used all available ECG and retinal fundus studies performed within the Yale New Haven Health System (YNHHS) from 2011- 2024 (train/validation/test split 80%/10%/10%). Separate convolutional neural network models (EfficientNetB3-based) were trained to predict age based on each modality [Figure A]. For individuals who had an ECG and retinal image within the same year, AI-age predictions were ensembled into a single cross-modal model. Predictive performance was evaluated using the mean absolute error (MAE) between predicted and chronological age in the test set. Individuals were categorized as ‘Resilient Agers’ if their predicted age was ≥1 MAE unit younger than their chronological age, ‘Accelerated Agers’ if ≥1 MAE unit older, and ‘Normal Agers’ otherwise. Cox proportional hazards models were fitted for chronic disease endpoints (hypertension, diabetes) as well as cardiovascular outcomes (MI, stroke, HF, death; together MACE) stratified by AI-aging phenotype. The study was externally validated in the UK Biobank.
RESULTS
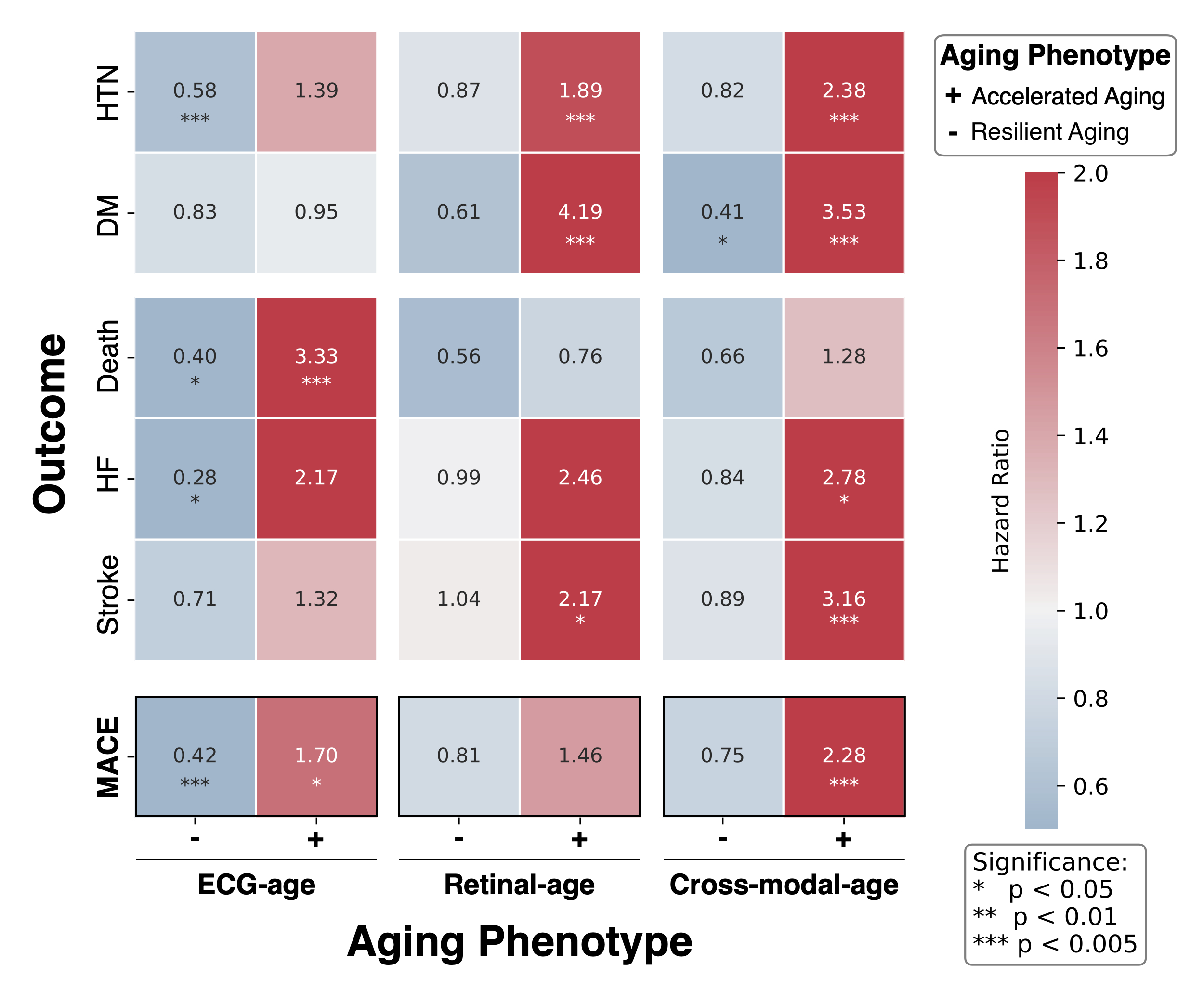
In the YNHHS cohort (n=195,851 patients, mean age 56 years [IQR 41-69]), ECG-age MAE was 9.1 and retinal-age MAE was 7.3, with similar performance in the validation set (ECG MAE 9.7; retinal MAE 7.5). Cross-modal AI-age predictions in paired studies (n =12,667, mean age 55 [43-69]) had a lower MAE of 5.9 years. The cross-modal Accelerated Ager phenotype was significantly associated with 10-year incidence of MACE (HR 2.28, CI [1.43, 3.66]), independent of chronological age, and with a larger effect size than for ECG- or retinal-age models. This pattern was also observed for individual MACE endpoints and risk factors [Figure B].
CONCLUSIONS
A unified ECG-retinal AI-age model delivers more accurate biologic-age estimates and identifies individuals whose 10-year MACE risk is doubled, independent of chronological age. This scalable, non-invasive framework offers a powerful tool for early cardiovascular risk stratification.
Age is the strongest factor in current cardiovascular risk estimation tools, yet there is significant heterogeneity in chronological age as a proxy for biological decline. Artificial intelligence (AI) models can infer biological age from routinely collected, non-invasive biomedical data. Here, we introduce a novel cross-modal AI-aging framework that leverages cardiac (ECG) and microvascular (retinal fundus images) aging phenotypes and its association with cardiovascular outcomes.
METHODS
For model development, we used all available ECG and retinal fundus studies performed within the Yale New Haven Health System (YNHHS) from 2011- 2024 (train/validation/test split 80%/10%/10%). Separate convolutional neural network models (EfficientNetB3-based) were trained to predict age based on each modality [Figure A]. For individuals who had an ECG and retinal image within the same year, AI-age predictions were ensembled into a single cross-modal model. Predictive performance was evaluated using the mean absolute error (MAE) between predicted and chronological age in the test set. Individuals were categorized as ‘Resilient Agers’ if their predicted age was ≥1 MAE unit younger than their chronological age, ‘Accelerated Agers’ if ≥1 MAE unit older, and ‘Normal Agers’ otherwise. Cox proportional hazards models were fitted for chronic disease endpoints (hypertension, diabetes) as well as cardiovascular outcomes (MI, stroke, HF, death; together MACE) stratified by AI-aging phenotype. The study was externally validated in the UK Biobank.
RESULTS
In the YNHHS cohort (n=195,851 patients, mean age 56 years [IQR 41-69]), ECG-age MAE was 9.1 and retinal-age MAE was 7.3, with similar performance in the validation set (ECG MAE 9.7; retinal MAE 7.5). Cross-modal AI-age predictions in paired studies (n =12,667, mean age 55 [43-69]) had a lower MAE of 5.9 years. The cross-modal Accelerated Ager phenotype was significantly associated with 10-year incidence of MACE (HR 2.28, CI [1.43, 3.66]), independent of chronological age, and with a larger effect size than for ECG- or retinal-age models. This pattern was also observed for individual MACE endpoints and risk factors [Figure B].
CONCLUSIONS
A unified ECG-retinal AI-age model delivers more accurate biologic-age estimates and identifies individuals whose 10-year MACE risk is doubled, independent of chronological age. This scalable, non-invasive framework offers a powerful tool for early cardiovascular risk stratification.
More abstracts on this topic:
Age-Related Differences in Aortic Valve Calcium Progression and the Risk for Aortic Stenosis: Multi-Ethnic Study of Atherosclerosis
Marrero Natalie, Thanassoulis George, Rotter Jerome, Blaha Michael, Whelton Seamus, Jha Kunal, Grant Jelani, Razavi Alexander, Budoff Matthew, Shah Sanjiv, Blumenthal Roger, Post Wendy, Shaw Leslee
Albuminuria Drives Type 2 Diabetes-Related Atrial Fibrillation: an ACCORD substudySiqueira Amanda, Everett Brendan