Final ID: 4366940
Phosphate Reduction During Hemodialysis Is Associated with Worsening Left Ventricular Global Longitudinal Strain
Abstract Body (Do not enter title and authors here): Introduction: Patients receiving hemodialysis (HD) experience higher mortality on dialysis days, implicating potential dialysis-specific factors. Phosphate is a critical component of ATP, and is rapidly removed with HD. We aimed to examine the relationship between the magnitude of phosphate reduction during HD and change in left ventricular strain measurements as a reflection of cardiac function.
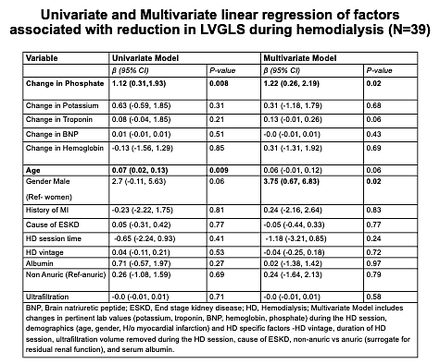
Methods: Prevalent ESKD patients on maintenance HD were prospectively recruited from a single center. Echocardiograms were performed pre- and mid-dialysis, with concurrent laboratory measurements including phosphate, potassium, troponin, brain natriuretic peptide, hemoglobin. Left ventricular global longitudinal strain (LVGLS), ejection fraction (LVEF), and global circumferential strain (LVGCS) were assessed. In those with worsening LVGLS (N=39), univariate and multivariate linear regression models assessed associations between changes in laboratory values and LVGLS adjusting for demographics, comorbidities, and HD-specific factors. Sensitivity analyses included adjustment for baseline LVEF and volume status (inferior vena cava measurements).
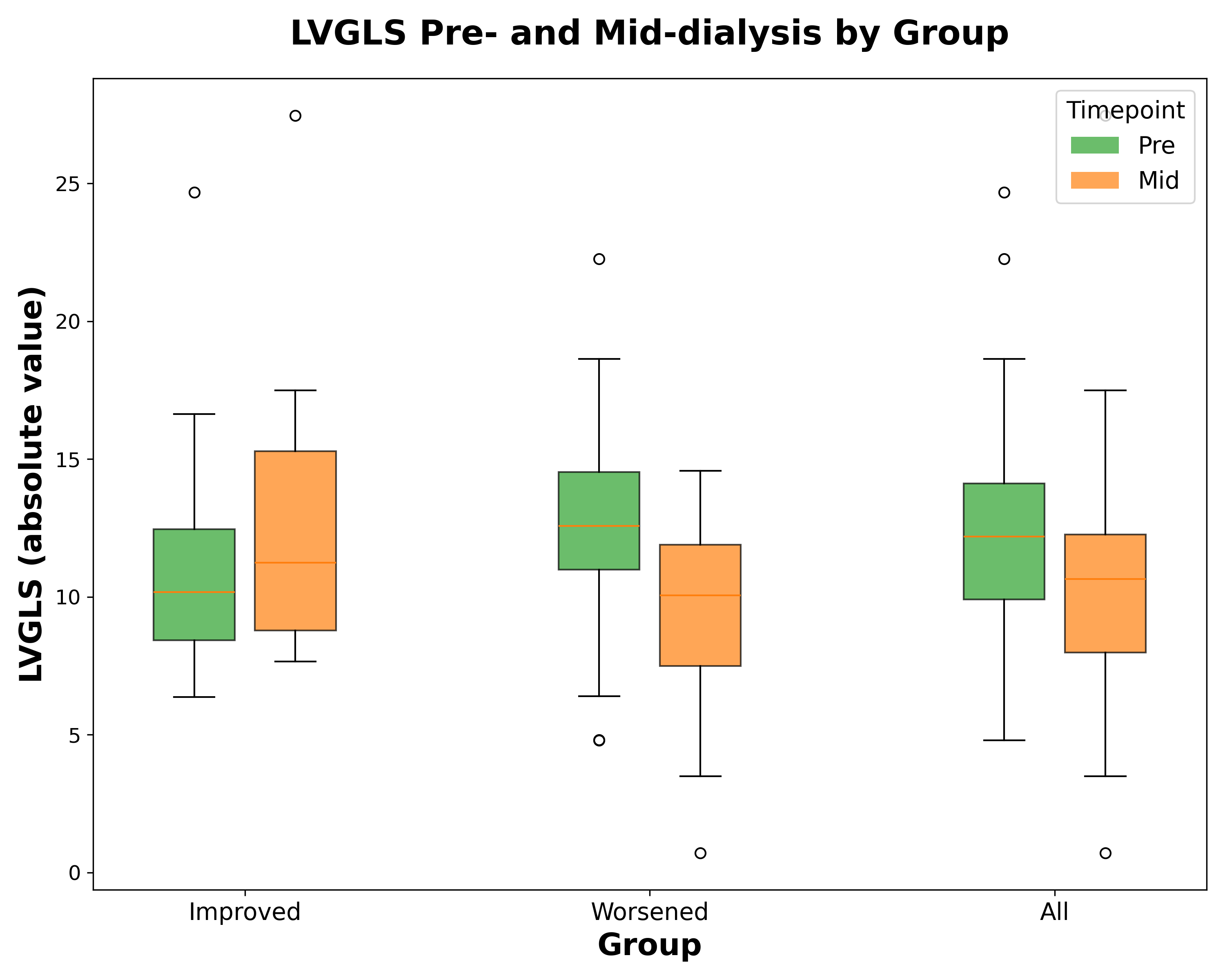
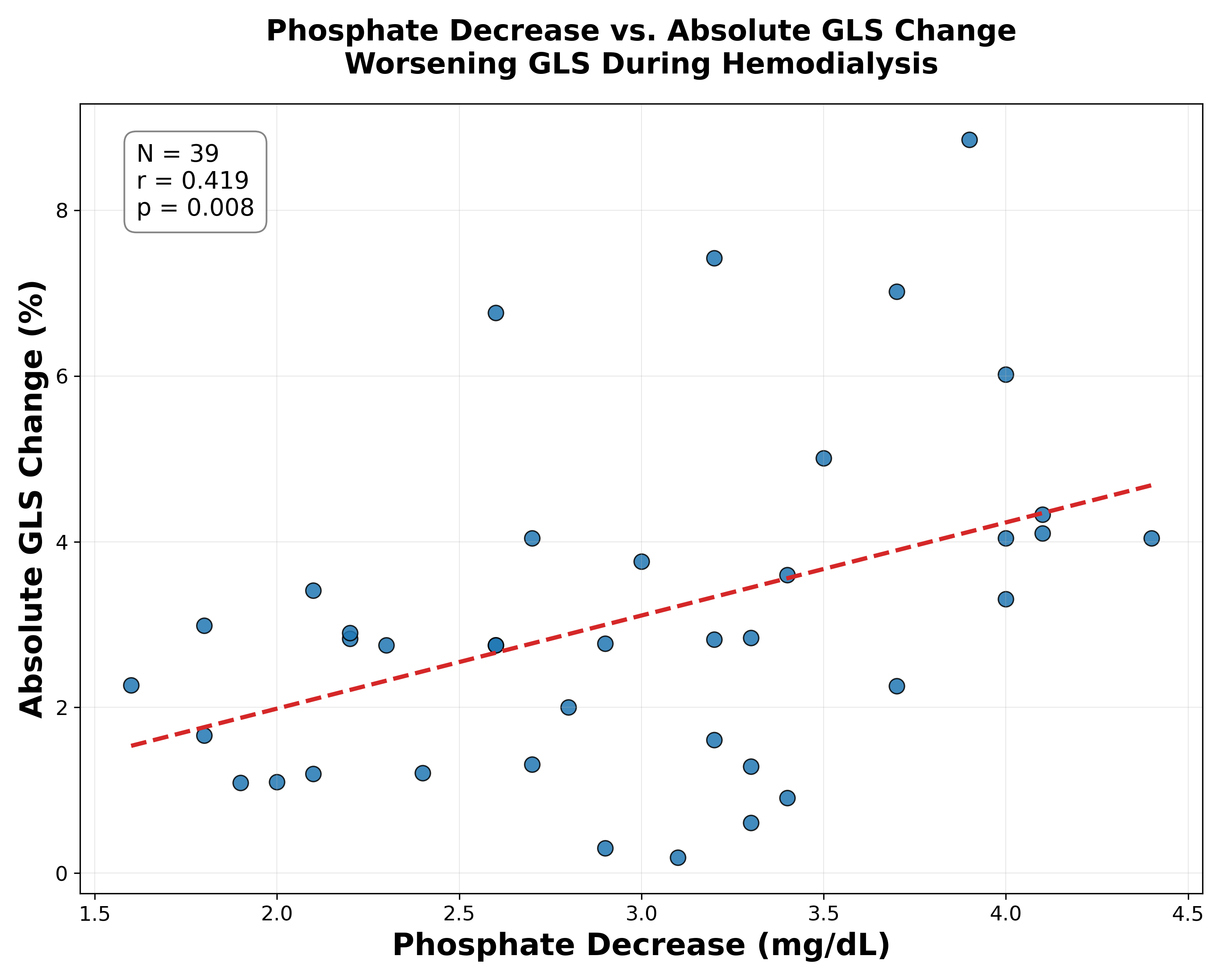
Results: The 54 HD participants had mean age 71 years, 96% male, reflective of a veterans-specific cohort. During HD, 75% (39/52) experienced worsening LVGLS (pre-HD: -12.64 ± 3.69, mid-HD: -9.56 ± 3.12, p<0.001), while 23% (12/52) improved (pre-HD: -11.35 ± 5.12, mid-HD: -12.79 ± 5.61, p<0.001). LVEF decreased overall (49.39% to 44.01%, p<0.001). No significant overall change was seen in LVGCS (mean 15.66 ± 4.49 to 15.54 ± 5.65). In multivariate models (Table 1), greater phosphate reduction was independently associated with worsening LVGLS (p<0.05), even after adjusting for changes in other labs, demographics, HD vintage, session duration, ultrafiltration volume, residual renal function, and albumin. Results were similar when adjusted for baseline LVEF, and volume status. Phosphate reduction was not significantly associated with changes in LVEF, LVGCS. Hs-troponin and BNP were not significantly altered.
Conclusion: Greater phosphate reduction during HD is independently associated with acute worsening of LVGLS, a sensitive marker of myocardial dysfunction, but not with changes in LVEF, LVGCS. These findings suggest that rapid phosphate shifts during HD may acutely contribute to adverse reduction in global myocardial deformation and highlight the need for further investigation into dialysis protocols that mitigate this risk.
Methods: Prevalent ESKD patients on maintenance HD were prospectively recruited from a single center. Echocardiograms were performed pre- and mid-dialysis, with concurrent laboratory measurements including phosphate, potassium, troponin, brain natriuretic peptide, hemoglobin. Left ventricular global longitudinal strain (LVGLS), ejection fraction (LVEF), and global circumferential strain (LVGCS) were assessed. In those with worsening LVGLS (N=39), univariate and multivariate linear regression models assessed associations between changes in laboratory values and LVGLS adjusting for demographics, comorbidities, and HD-specific factors. Sensitivity analyses included adjustment for baseline LVEF and volume status (inferior vena cava measurements).
Results: The 54 HD participants had mean age 71 years, 96% male, reflective of a veterans-specific cohort. During HD, 75% (39/52) experienced worsening LVGLS (pre-HD: -12.64 ± 3.69, mid-HD: -9.56 ± 3.12, p<0.001), while 23% (12/52) improved (pre-HD: -11.35 ± 5.12, mid-HD: -12.79 ± 5.61, p<0.001). LVEF decreased overall (49.39% to 44.01%, p<0.001). No significant overall change was seen in LVGCS (mean 15.66 ± 4.49 to 15.54 ± 5.65). In multivariate models (Table 1), greater phosphate reduction was independently associated with worsening LVGLS (p<0.05), even after adjusting for changes in other labs, demographics, HD vintage, session duration, ultrafiltration volume, residual renal function, and albumin. Results were similar when adjusted for baseline LVEF, and volume status. Phosphate reduction was not significantly associated with changes in LVEF, LVGCS. Hs-troponin and BNP were not significantly altered.
Conclusion: Greater phosphate reduction during HD is independently associated with acute worsening of LVGLS, a sensitive marker of myocardial dysfunction, but not with changes in LVEF, LVGCS. These findings suggest that rapid phosphate shifts during HD may acutely contribute to adverse reduction in global myocardial deformation and highlight the need for further investigation into dialysis protocols that mitigate this risk.
More abstracts on this topic:
A Diagnosis Dilemma of Positional Hypoxia: Scoliosis-Mediated Platypnea-Orthodeoxia Syndrome
Ademuwagun Christianah, Arjoon Roy, Seth Paula, Chang Gene, Ibe Oby
A Hemodynamic Warning Sign: Continuous Mitral Regurgitation and Normal Sinus RhythmMahi Ishani, Chowdhury Mahdi, Madan Hritik, Garg Vaani