Final ID: Mo3012
Combined Efficacy, Safety, and Test Dose Tolerability of Etripamil for Acute Paroxysmal Supraventricular Tachycardia (PSVT) Across Multiple Clinical Trials
Abstract Body (Do not enter title and authors here): Background: Etripamil nasal spray is a fast-acting, calcium channel blocker in development for the conversion of paroxysmal supraventricular tachycardia (PSVT) outside of a health-care setting. Studies have demonstrated the favorable safety and efficacy of self-administered etripamil in restoring sinus rhythm (SR) and reducing emergency department visits.
Purpose: This report analyzes the efficacy, safety, and test-dose tolerability of etripamil across PSVT clinical studies.
Methods: We systemically examined randomized, controlled and open-label trials evaluating etripamil in patients with PSVT. Efficacy measures included the proportion of patients converting from PSVT to SR within 30 minutes and the median times to conversion across all Phase 3 studies. Safety data for treatment-emergent adverse events (TEAEs), serious AEs, and test dose tolerability from Phase 2/3 studies were summarized descriptively.
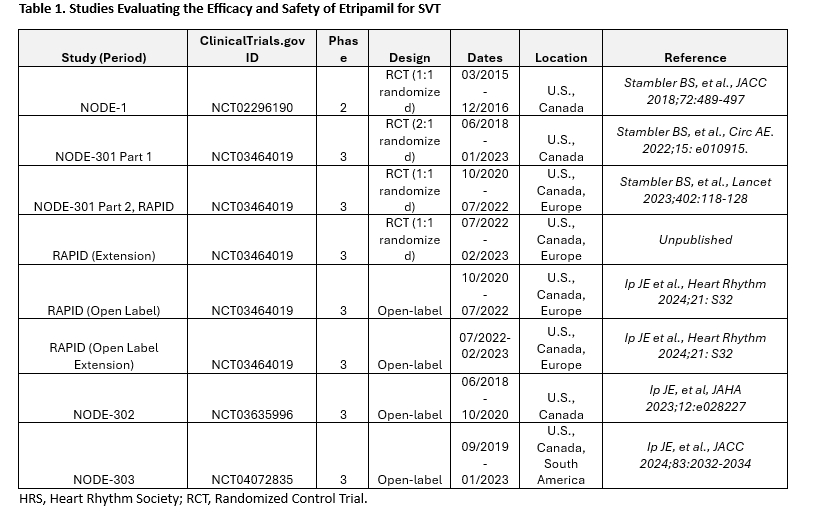
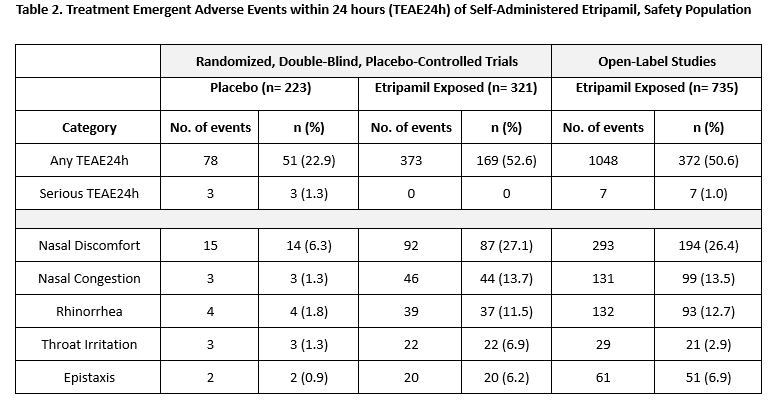
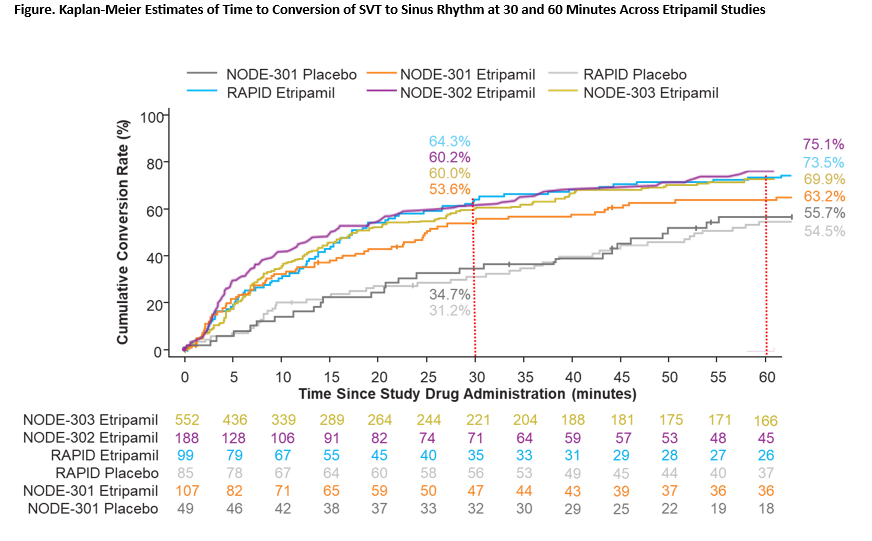
Results: NODE-1, NODE-301 Part 1, NODE-301 Part 2 (RAPID, including RAPID Extension, RAPID Open Label, RAPID Open Label Extension periods), NODE-302, and NODE-303 studies were analyzed (Table 1). The Kaplan-Meier estimate of etripamil exposed patients converting to SR within 30 minutes (n= 622) was 59.6% (range, 53.6% to 64.3%) with a median time to conversion of 18.5 minutes (95% CI, 15.7 to 21.0). The proportions of RCT placebo group patients converting from SVT to SR by 30 minutes ranged from 26.7% to 34.7%. Estimates of conversion of SVT to SR with etripamil at 60 minutes for individual studies ranged from 63.2% to 73.5% (Figure). Combined analysis showed mainly the occurrence of mild, transient TEAEs, primarily nasal discomfort, nasal congestion, rhinorrhea, throat irritation, and epistaxis (Table 2). 1,107 patients took at least one etripamil test dose while in SR; no significant change in average baseline heart rate or blood pressure was observed within 45 minutes of administration, and test dose failure occurred in only 16 patients (1.4%).
Conclusions: Across multiple clinical studies of acute PSVT management, self-administered etripamil consistently demonstrated robust efficacy and a favorable safety profile for acute PSVT management. Safety data and the low rate of test dose failures indicate favorable tolerability, suggesting no need for a pretreatment test dose. These findings support the potential of etripamil as a patient-administered therapy for PSVT, which may reduce reliance on emergency care.
Purpose: This report analyzes the efficacy, safety, and test-dose tolerability of etripamil across PSVT clinical studies.
Methods: We systemically examined randomized, controlled and open-label trials evaluating etripamil in patients with PSVT. Efficacy measures included the proportion of patients converting from PSVT to SR within 30 minutes and the median times to conversion across all Phase 3 studies. Safety data for treatment-emergent adverse events (TEAEs), serious AEs, and test dose tolerability from Phase 2/3 studies were summarized descriptively.
Results: NODE-1, NODE-301 Part 1, NODE-301 Part 2 (RAPID, including RAPID Extension, RAPID Open Label, RAPID Open Label Extension periods), NODE-302, and NODE-303 studies were analyzed (Table 1). The Kaplan-Meier estimate of etripamil exposed patients converting to SR within 30 minutes (n= 622) was 59.6% (range, 53.6% to 64.3%) with a median time to conversion of 18.5 minutes (95% CI, 15.7 to 21.0). The proportions of RCT placebo group patients converting from SVT to SR by 30 minutes ranged from 26.7% to 34.7%. Estimates of conversion of SVT to SR with etripamil at 60 minutes for individual studies ranged from 63.2% to 73.5% (Figure). Combined analysis showed mainly the occurrence of mild, transient TEAEs, primarily nasal discomfort, nasal congestion, rhinorrhea, throat irritation, and epistaxis (Table 2). 1,107 patients took at least one etripamil test dose while in SR; no significant change in average baseline heart rate or blood pressure was observed within 45 minutes of administration, and test dose failure occurred in only 16 patients (1.4%).
Conclusions: Across multiple clinical studies of acute PSVT management, self-administered etripamil consistently demonstrated robust efficacy and a favorable safety profile for acute PSVT management. Safety data and the low rate of test dose failures indicate favorable tolerability, suggesting no need for a pretreatment test dose. These findings support the potential of etripamil as a patient-administered therapy for PSVT, which may reduce reliance on emergency care.
More abstracts on this topic:
Impact of Supraventricular Tachycardia on Pregnancy Outcomes
Ardehali Arya, Kiess Marla, Rychel Valerie, Barlow Amanda, Oakes Jennifer, Deyell Marc, Grewal Jasmine
A Case of Dilated Cardiomyopathy and Systemic Thromboembolism in a Young Patient on Testosterone Replacement TherapySabri Muhammad, Ijaz Naila, Nadeem Ramsha, Checchio Lucy, Riaz Faiza