Final ID: MDP1250
Frequency and Patterns of Paroxysmal Supraventricular Tachycardia Episodes Among Patients Opting For Acute Drug Treatment: Analysis of the NODE-303 Open-Label Etripamil Trial
Abstract Body (Do not enter title and authors here): Background: Etripamil nasal spray (NS) is a fast acting, self-administered calcium channel blocker in development for the termination of AV-nodal-dependent supraventricular tachycardia (SVT). Prior randomized, placebo-controlled and open-label studies have demonstrated favorable safety and efficacy of etripamil in converting paroxysmal supraventricular tachycardia (PSVT) to sinus rhythm (SR) self-administered without direct medical supervision.
Research Question/Hypothesis: To assess patterns and annualized PSVT episode frequency among patients opting to self-administer acute treatment with etripamil.
Methods: NODE-303 was an event-driven, multi-center, open-label Phase 3 study, conducted in North and South America to evaluate the safety and efficacy of etripamil in patients with documented PSVT over multiple episodes. Test dosing was not performed prior to at-home use. Enrolled patients, upon perceiving symptoms of PSVT: applied an ambulatory ECG monitor, performed a previously trained vagal maneuver and, if symptoms persisted, self-administered etripamil NS 70 mg. During the study, the protocol was amended to allow a repeat dose (70 mg) if symptoms persisted 10 min after the first dose. Each patient could self-treat up to 4 episodes.
Results:
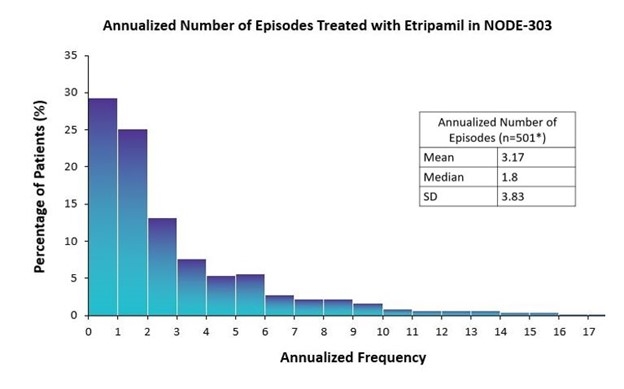
Of 1,116 enrolled patients, 503 (45.1%) treated ≥1 perceived PSVT episode (safety population). Etripamil achieved conversion to SR in 60% of patients by 30 minutes and 70% by 60 minutes. A total of 220, 118, 62, and 103 patients completed the study with 1, 2, 3, and 4 etripamil-treated perceived episodes of PSVT, respectively, with an average time on study of 440 days. Among these patients, the average number of annualized etripamil-treated PSVT episodes was 3.2 (standard deviation 3.8). Annualized use: etripamil was self-administered for 0-2 PSVT episodes per year, 2-6 episodes, 6-12 episodes, and >12 episodes, in 54%, 32%, 10%, and 4% of patients, respectively (Figure).
Conclusions:
This analysis aimed to assess the annualized use of etripamil NS in a real-world setting, by analyzing how often patients would self-administer the drug for PSVT episodes. Of patients that self-administered etripamil (n=503), the majority treated >1 episode and the annualized frequency of episodes treated with etripamil was 3.2 episodes/yr.
Research Question/Hypothesis: To assess patterns and annualized PSVT episode frequency among patients opting to self-administer acute treatment with etripamil.
Methods: NODE-303 was an event-driven, multi-center, open-label Phase 3 study, conducted in North and South America to evaluate the safety and efficacy of etripamil in patients with documented PSVT over multiple episodes. Test dosing was not performed prior to at-home use. Enrolled patients, upon perceiving symptoms of PSVT: applied an ambulatory ECG monitor, performed a previously trained vagal maneuver and, if symptoms persisted, self-administered etripamil NS 70 mg. During the study, the protocol was amended to allow a repeat dose (70 mg) if symptoms persisted 10 min after the first dose. Each patient could self-treat up to 4 episodes.
Results:
Of 1,116 enrolled patients, 503 (45.1%) treated ≥1 perceived PSVT episode (safety population). Etripamil achieved conversion to SR in 60% of patients by 30 minutes and 70% by 60 minutes. A total of 220, 118, 62, and 103 patients completed the study with 1, 2, 3, and 4 etripamil-treated perceived episodes of PSVT, respectively, with an average time on study of 440 days. Among these patients, the average number of annualized etripamil-treated PSVT episodes was 3.2 (standard deviation 3.8). Annualized use: etripamil was self-administered for 0-2 PSVT episodes per year, 2-6 episodes, 6-12 episodes, and >12 episodes, in 54%, 32%, 10%, and 4% of patients, respectively (Figure).
Conclusions:
This analysis aimed to assess the annualized use of etripamil NS in a real-world setting, by analyzing how often patients would self-administer the drug for PSVT episodes. Of patients that self-administered etripamil (n=503), the majority treated >1 episode and the annualized frequency of episodes treated with etripamil was 3.2 episodes/yr.
More abstracts on this topic:
Intravenous sotalol in pediatric cardiac intensive care patients compared to other antiarrhythmics: insights from a multicenter database
Loomba Rohit, Backes Emily, Farias Juan, Borquez Alejandro, Malloy-walton Lindsey, Flores Saul
Adverse Outcomes Following Atrial Fibrillation Ablation in Patients With Connective Tissue Disease: A Propensity-Matched Real-World AnalysisRayyan Abdallah, Obeidat Omar, Alqudah Qusai, Khasawneh Tawfiq, Alomari Ahmad, Mestarihi Aseed, Alnabahneh Nizar, Nasser Hesham, Tong Ann