Final ID: MP2507
Risk Stratification for Cancer Therapy-Related Cardiac Dysfunction: External Validation of a HER2+ Breast Cancer Nomogram
Abstract Body (Do not enter title and authors here): Background
Cancer therapy–related cardiac dysfunction (CTRCD) is a well-recognized adverse effect of HER2-targeted therapies, particularly trastuzumab. While current guidelines recommend serial left ventricular ejection fraction (LVEF) monitoring every 3 months during treatment, such uniform surveillance may lead to overutilization in low-risk patients.
Objective: This study aimed to externally validate a nomogram from Memorial Sloan Kettering to predict CTRCD in a contemporary cohort of patients with HER2-positive breast cancer.
Methods
This retrospective study included women with HER2-positive breast cancer treated with trastuzumab between 2013 & 2022 at a large cancer center. The primary endpoint was 1-year CTRCD-free survival, with CTRCD defined as an LVEF decline ≥10% to <53% or a ≥16% reduction from baseline. Patients were stratified into risk groups based on total nomogram points. Model performance was evaluated in the validation cohort using Kaplan-Meier estimates, calibration plots, concordance index (C-index), and area under the ROC curve (AUC).
Results
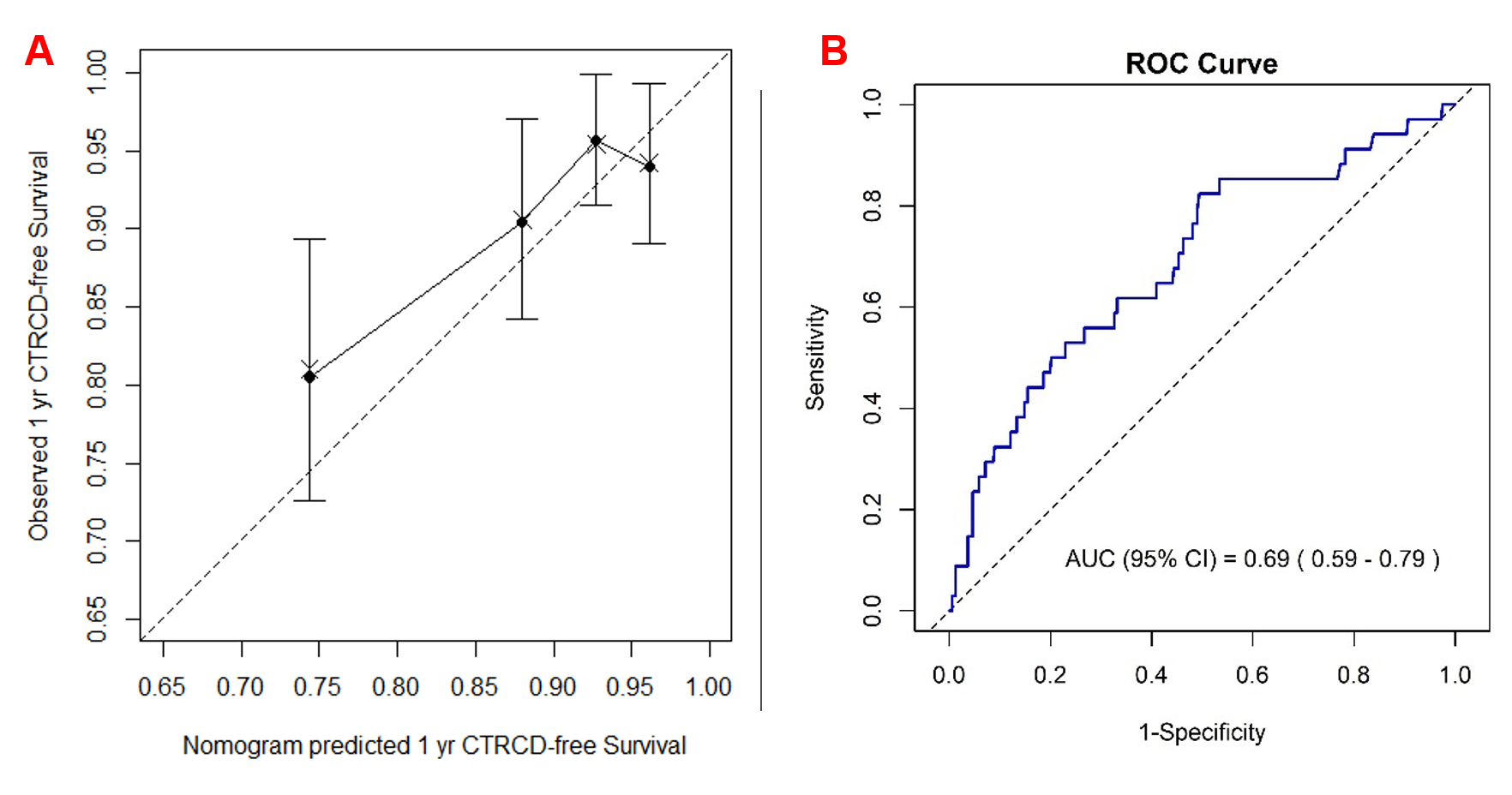
The derivation and validation cohorts demonstrated notable differences in baseline characteristics. Patients in the validation cohort (n=356) had a higher mean BMI (39.6% vs. 22.9%) and greater prevalence of cardiovascular comorbidities, including hypertension (34.8% vs. 23.5%), diabetes (11.5% vs. 7.2%), and hyperlipidemia (24.7% vs. 17.1%) but with substantially lower anthracycline exposure (31.5% vs. 77.8%) compared to the derivation cohort. 9.6% of patients developed CTRCD within 395 days of trastuzumab initiation, which was defined as the 1-year CTRCD event. The 1-year CTRCD event rates across increasing quartiles of the nomogram score were 6.0%, 4.4%, 9.6%, and 19.5%, respectively, indicating effective risk stratification. Kaplan-Meier–estimated 1-year CTRCD-free survival was 90.1%, closely aligned with the nomogram-predicted probability of 87.8%. The model demonstrated excellent discriminative performance, with a C-index of 0.68 (95% CI, 0.49–0.86) and an AUC of 0.69 (95% CI, 0.58–0.78) (Figure A, B).
Conclusion
The novel CTRCD nomogram demonstrated excellent discriminatory power and generalizable to an independent, real-world patient population. These findings support its utility for personalized CTRCD risk stratification and suggest its potential to guide less frequent LVEF monitoring in low-risk patients receiving HER2-targeted therapy.
Cancer therapy–related cardiac dysfunction (CTRCD) is a well-recognized adverse effect of HER2-targeted therapies, particularly trastuzumab. While current guidelines recommend serial left ventricular ejection fraction (LVEF) monitoring every 3 months during treatment, such uniform surveillance may lead to overutilization in low-risk patients.
Objective: This study aimed to externally validate a nomogram from Memorial Sloan Kettering to predict CTRCD in a contemporary cohort of patients with HER2-positive breast cancer.
Methods
This retrospective study included women with HER2-positive breast cancer treated with trastuzumab between 2013 & 2022 at a large cancer center. The primary endpoint was 1-year CTRCD-free survival, with CTRCD defined as an LVEF decline ≥10% to <53% or a ≥16% reduction from baseline. Patients were stratified into risk groups based on total nomogram points. Model performance was evaluated in the validation cohort using Kaplan-Meier estimates, calibration plots, concordance index (C-index), and area under the ROC curve (AUC).
Results
The derivation and validation cohorts demonstrated notable differences in baseline characteristics. Patients in the validation cohort (n=356) had a higher mean BMI (39.6% vs. 22.9%) and greater prevalence of cardiovascular comorbidities, including hypertension (34.8% vs. 23.5%), diabetes (11.5% vs. 7.2%), and hyperlipidemia (24.7% vs. 17.1%) but with substantially lower anthracycline exposure (31.5% vs. 77.8%) compared to the derivation cohort. 9.6% of patients developed CTRCD within 395 days of trastuzumab initiation, which was defined as the 1-year CTRCD event. The 1-year CTRCD event rates across increasing quartiles of the nomogram score were 6.0%, 4.4%, 9.6%, and 19.5%, respectively, indicating effective risk stratification. Kaplan-Meier–estimated 1-year CTRCD-free survival was 90.1%, closely aligned with the nomogram-predicted probability of 87.8%. The model demonstrated excellent discriminative performance, with a C-index of 0.68 (95% CI, 0.49–0.86) and an AUC of 0.69 (95% CI, 0.58–0.78) (Figure A, B).
Conclusion
The novel CTRCD nomogram demonstrated excellent discriminatory power and generalizable to an independent, real-world patient population. These findings support its utility for personalized CTRCD risk stratification and suggest its potential to guide less frequent LVEF monitoring in low-risk patients receiving HER2-targeted therapy.
More abstracts on this topic:
A Novel Echocardiography Risk Score Predicted Mortality In Patients With Heart Failure With Preserved Ejection Fraction.
Iwakura Katsuomi, Yoshio Yasumura, Hikoso Shungo, Okada Katsuki, Nakatani Daisaku, Sotomi Yohei, Sakata Yasushi, Tanaka Nobuaki, Okada Masato, Okamura Atsunori, Heitaro Watanabe, Seo Masahiro, Hayashi Takaharu, Yano Masamichi, Yamada Takahisa
A Case Presentation of Severe Left Ventricular Dysfunction from Focal Myocarditis due to Immune Checkpoint InhibitorPatel Romil, Hussain Kifah, Gordon Robert