Final ID: MP2222
Major Adverse Cardiovascular Events across the Spectrum of Cardio-Kidney-Metabolic Syndrome: A FINE-HEART Pooled Analysis
Mineralocorticoid receptor antagonists (MRAs) mechanistically reduce inflammation, oxidative stress and endothelial dysfunction. There is growing interest in understanding the composite cardiovascular (CV) protection afforded by therapies like the nonsteroidal MRA finerenone with systemic actions in patients with cardio-kidney-metabolic (CKM) syndrome.
METHODS:
In this participant-level pre-specified pooled analysis from three large phase 3 clinical trials (FIDELIO-DKD, FIGARO-DKD, and FINEARTS-HF), we assessed the association between various nonfatal CV events (myocardial infarction, stroke, and heart failure hospitalization) and rates of subsequent mortality using time-updated models. We then examined the treatment effects of finerenone vs. placebo on major adverse cardiovascular events (MACE, a composite of CV death, nonfatal myocardial infarction, nonfatal stroke, or heart failure hospitalization), which was a prespecified secondary endpoint in the FINE-HEART pooled analysis, using Cox regression models stratified by trial and region.
RESULTS:
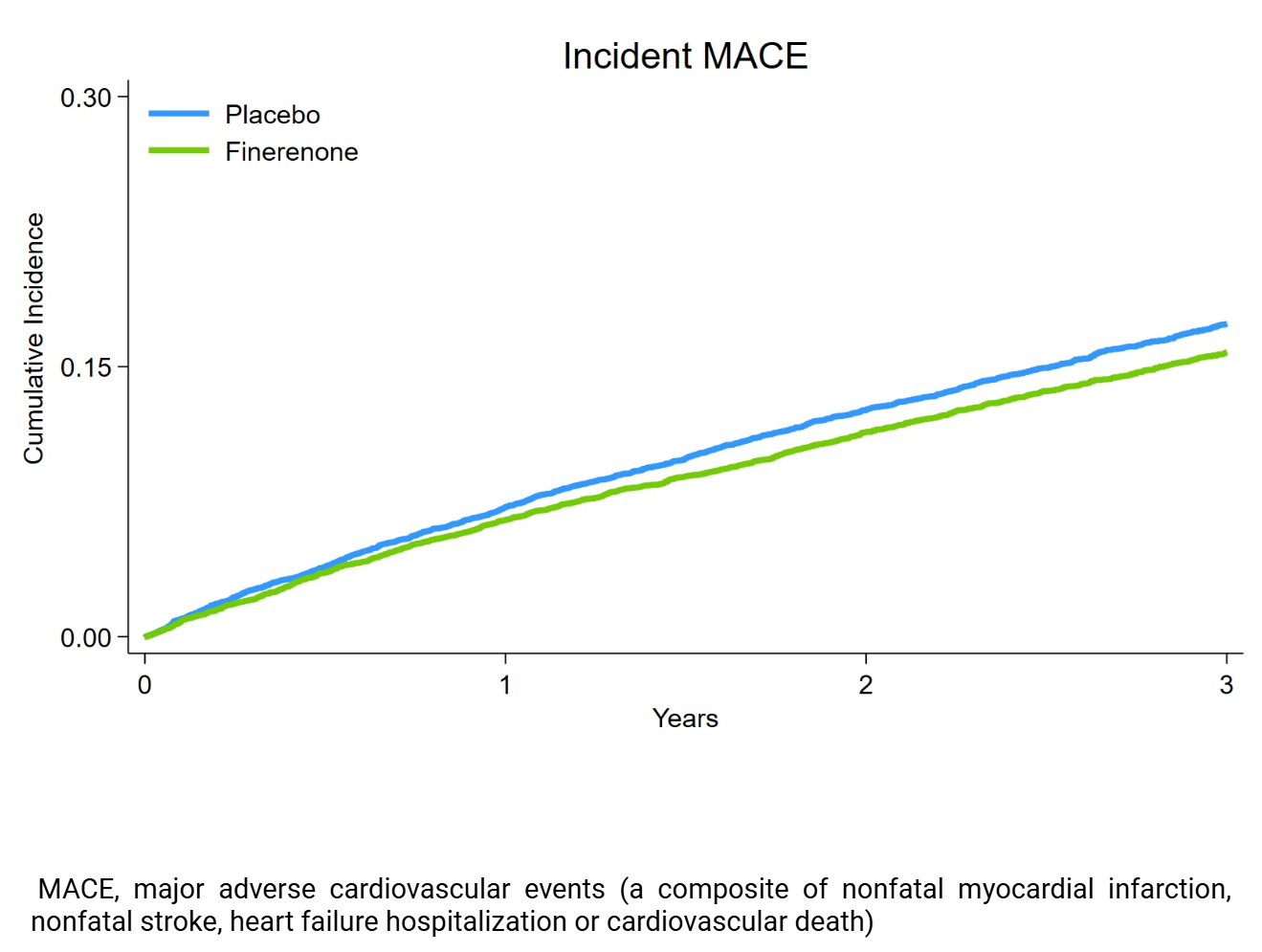
During a median of 2.9 years of follow-up, among the 18,991 participants, 1,544 (8.1%) experienced heart failure hospitalization, 500 (2.6%) nonfatal myocardial infarction, 570 (3.0%) nonfatal stroke, and 892 (4.7%) CV death. Patients with incident myocardial infarction, stroke, and heart failure hospitalization consistently experienced markedly higher subsequent risks of mortality (Figure 1). Mortality was highest after heart failure hospitalization (incidence rate 23.4 [21.4-25.8] per 100py compared with 3.2 [3.1-3.4] per 100py for individuals without nonfatal CV events). Finerenone reduced the composite of CV death, nonfatal myocardial infarction, nonfatal stroke, or heart failure hospitalization (HR 0.91; 95% CI, 0.85–0.98; P = 0.010, Figure 2). Results were essentially unchanged in a sensitivity analysis including undetermined deaths as CV deaths (HR 0.90; 95% CI, 0.84–0.96; P = 0.002). The treatment effect on MACE was consistent across FINEARTS-HF (HR 0.95; 95% CI 0.86–1.05), FIDELIO-DKD (HR 0.88; 95% CI 0.76–1.02), and FIGARO-DKD (HR 0.87; 95% CI 0.76–1.00); Pint=0.55. Risk reductions did not differ by the number of CKM conditions (Pint=0.98).
CONCLUSION
Among patients with cardio-kidney-metabolic syndrome, major adverse cardiovascular events were frequent, prognostically meaningful, and reduced with the non-steroidal MRA finerenone.
- Siqueira, Sara ( Cardiovascular Division, Brigham And Women's Hospital, Harvard Medical School , Boston , Massachusetts , United States )
- Brinker, Meike ( Bayer AG, Research & Development, Pharmaceuticals , Wuppertal , Germany )
- Lay-flurrie, James ( Bayer plc, Research & Development, Pharmaceuticals , Reading , United Kingdom )
- Rohwedder, Katja ( Bayer AG, Global Medical Affairs , Berlin , Germany )
- Lam, Carolyn ( National Heart Centre Singapore and Duke-National University of Singapore , Singapore , Singapore )
- Senni, Michele ( University of Milano-Bicocca ASST Papa Giovanni XXIII Hospital , Bergamo , Italy )
- Shah, Sanjiv ( Feinberg Cardiovascular Research Institute, Northwestern University Feinberg School of Medicine , Chicago , Illinois , United States )
- Voors, Adriaan ( University Medical Center Groningen , Groningen , Netherlands )
- Zannad, Faiez ( Université de Lorraine, Inserm Clinical Investigation Centre, CHU , Nancy , France )
- Rossing, Peter ( Steno Diabetes Center Copenhagen and University of Copenhagen , Copenhagen , Denmark )
- Ruilope, Luis ( Hospital 12 de Octubre , Madrid , Spain )
- Pabon, Maria ( Cardiovascular Division, Brigham And Women's Hospital, Harvard Medical School , Boston , Massachusetts , United States )
- Anker, Stefan ( Department of Cardiology (CVK) of German Heart Center Charité, German Centre for Cardiovascular Research (DZHK) Partner Site Berlin, Charité Universitätsmedizin , Berlin , Germany )
- Pitt, Bertram ( University of Michigan , Ann Arbor , Michigan , United States )
- Agarwal, Rajiv ( Indiana University School of Medicine , Indianapolis , Indiana , United States )
- Mcmurray, John ( British Heart Foundation Cardiovascular Research Centre, School of Cardiovascular and Metabolic Health, University of Glasgow , Glasgow , United Kingdom )
- Solomon, Scott ( Cardiovascular Division, Brigham And Women's Hospital, Harvard Medical School , Boston , Massachusetts , United States )
- Vaduganathan, Muthiah ( Cardiovascular Division, Brigham And Women's Hospital, Harvard Medical School , Boston , Massachusetts , United States )
- Makuvire, Tracy ( Cardiovascular Division, Brigham And Women's Hospital, Harvard Medical School , Boston , Massachusetts , United States )
- Claggett, Brian ( Cardiovascular Division, Brigham And Women's Hospital, Harvard Medical School , Boston , Massachusetts , United States )
- Filippatos, Gerasimos ( National and Kapodistrian University of Athens, School of Medicine, Attikon University Hospital , Athens , Greece )
- Chatur, Safia ( Massachusetts General Hospital, Harvard Medical School , Boston , Massachusetts , United States )
- Desai, Akshay ( Cardiovascular Division, Brigham And Women's Hospital, Harvard Medical School , Boston , Massachusetts , United States )
- Jhund, Pardeep ( British Heart Foundation Cardiovascular Research Centre, School of Cardiovascular and Metabolic Health, University of Glasgow , Glasgow , United Kingdom )
- Henderson, Alasdair David ( British Heart Foundation Cardiovascular Research Centre, School of Cardiovascular and Metabolic Health, University of Glasgow , Glasgow , United Kingdom )
Meeting Info:
Session Info:
Interventional Studies and Outcome Trends in CKM Syndrome
Monday, 11/10/2025 , 12:15PM - 01:25PM
Moderated Digital Poster Session
More abstracts on this topic:
Shahid Abdulla, Arora Pankaj, Pampana Akhil, Gaonkar Mokshad, Patel Nirav, Bal Harshvir, Nayak Amrita, Vekariya Nehal, Shetty Naman, Arora Garima
An ADPKD-Associated Pathway in Cardiac Homeostasis, Heart Failure, and Cardiovascular-kidney-metabolicLiu Chia-feng, Leon Steven, Wessely Oliver, Tang Wai Hong
More abstracts from these authors:
Inciardi Riccardo, Scalise Andrea, Lam Carolyn, Senni Michele, Shah Sanjiv, Voors Adriaan, Zannad Faiez, Rossing Peter, Ruilope Luis, Anker Stefan, Pitt Bertram, Ostrominski John, Agarwal Rajiv, Mcmurray John, Solomon Scott, Vaduganathan Muthiah, Claggett Brian, Desai Akshay, Jhund Pardeep, Henderson Alasdair David, Brinker Meike, Lay-flurrie James, Glasauer Andrea
Timing of Cardiovascular and Kidney Benefits with Finerenone in Heart Failure and Chronic Kidney Disease with Type 2 DiabetesOstrominski John, Shah Sanjiv, Voors Adriaan, Zannad Faiez, Rossing Peter, Ruilope Luis, Anker Stefan, Pitt Bertram, Agarwal Rajiv, Lay-flurrie James, Brinker Meike, Neuen Brendon, Amarante Flaviana, Hofmeister Lucas, Mcmurray John, Solomon Scott, Vaduganathan Muthiah, Claggett Brian, Filippatos Gerasimos, Desai Akshay, Jhund Pardeep, Henderson Alasdair David, Lam Carolyn, Senni Michele