Final ID: MP2232
Timing of Cardiovascular and Kidney Benefits with Finerenone in Heart Failure and Chronic Kidney Disease with Type 2 Diabetes
Background: The nonsteroidal mineralocorticoid receptor antagonist finerenone has been shown to reduce adverse clinical outcomes in persons with heart failure (HF) and chronic kidney disease (CKD) with type 2 diabetes. However, the relative timing of these benefits has not been evaluated.
Research Question: What is the timing of cardiovascular and kidney protection with finerenone?
Methods: In this participant-level analysis of randomized, placebo-controlled, phase 3 outcomes trials evaluating finerenone in persons with HF (FINEARTS-HF) and CKD with type 2 diabetes (FIGARO-DKD and FIDELIO-DKD [FIDELITY]), we evaluated the cumulative number and type of clinical outcomes (time-to-first) prevented over time post-randomization. The timing of first statistically significant benefit of finerenone on selected clinical outcomes was additionally assessed. Estimates of events prevented (per 10,000 treated participants) were calculated using the difference in observed events between treatment arms, and were displayed graphically.
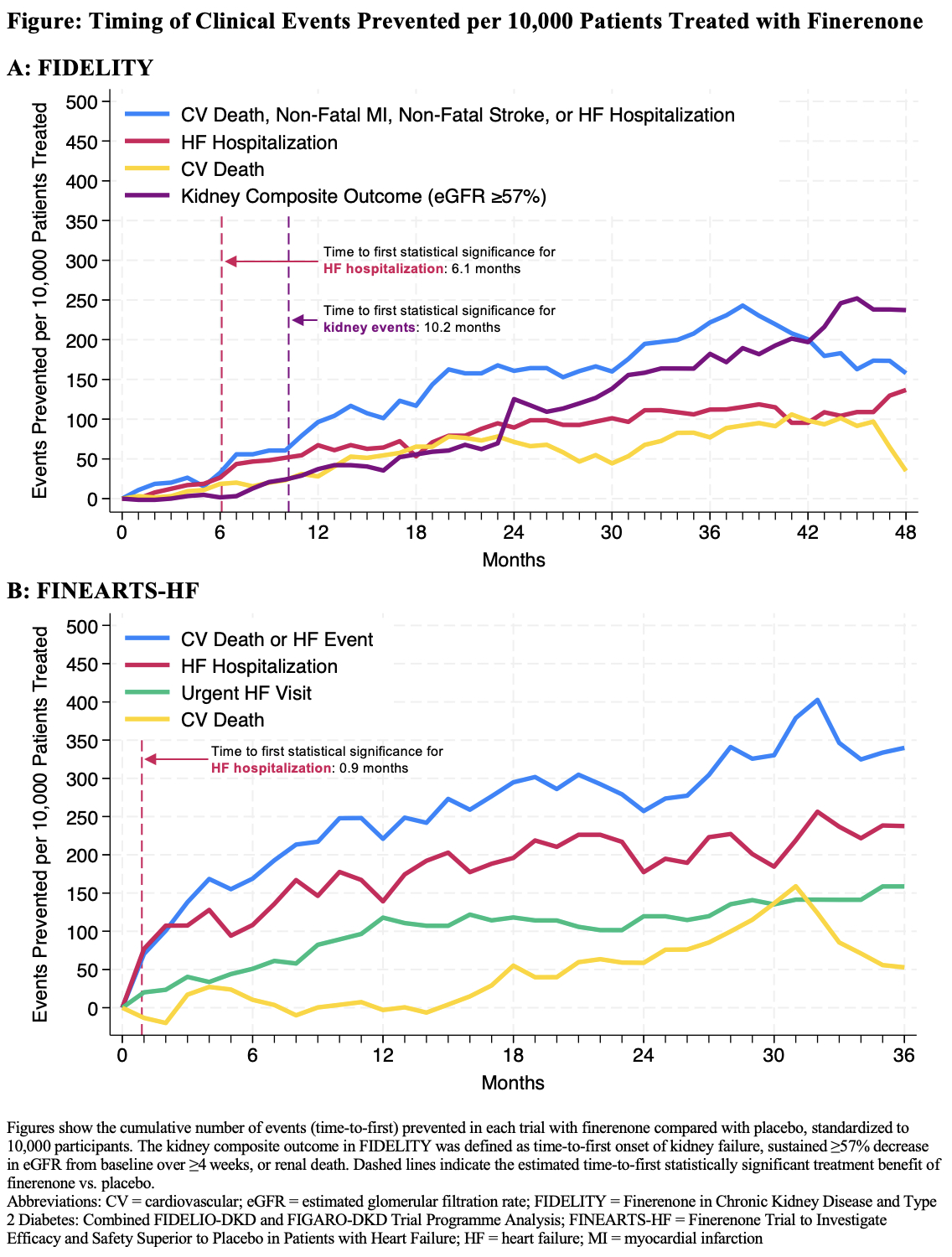
Results: In both trial populations, benefits with finerenone occurred early and accrued over time (Figure). In both FIDELITY and FINEARTS-HF, HF hospitalization appeared to be the earliest type of event prevented. In FIDELITY, time to first nominal statistical significance for HF hospitalization was 6.1 months (HR, 0.65; 95% CI, 0.43 to 0.999), compared with 10.2 months (HR, 0.59; 95% CI, 0.35 to 0.98) for the composite kidney outcome (Figure). Similarly, first statistical significance for HF hospitalization in FINEARTS-HF was attained after 0.9 months (HR, 0.55; 95% CI, 0.32 to 0.96). At 12 months, we estimated 67 and 140 HF hospitalizations per 10,000 patients would be prevented with finerenone vs. placebo, based on findings from FIDELITY and FINEARTS-HF, respectively (Figure). While kidney benefits in FIDELITY were initially slower to accumulate compared with cardiovascular benefits, the number of kidney events prevented (216 per 10,000 patients) exceeded the number of cardiovascular events prevented (180 per 10,000 patients) by 43 months (Figure).
Conclusions: These findings underscore the high short-term risks of HF events in persons with cardiovascular, kidney, and/or metabolic conditions, which are modifiable with finerenone. When used in CKD management, finerenone prevents cardiovascular events even prior to modifying longer-term risks of kidney disease progression.
- Ostrominski, John ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Shah, Sanjiv ( NORTHWESTERN UNIVERSITY , Chicago , Illinois , United States )
- Voors, Adriaan ( UNIVERSITY MEDICAL CENTER GRONINGEN , Gronien , Netherlands )
- Zannad, Faiez ( CVCT and Universite de Lorraine , Paris , France )
- Rossing, Peter ( Steno Diabetes Center Copenhagen , Gentofte , Denmark )
- Ruilope, Luis ( Hospital 12 de Octubre , Madrid , Spain )
- Anker, Stefan ( charite , Berlin , Germany )
- Pitt, Bertram ( University of Michigan School , Ann Arbor , Michigan , United States )
- Agarwal, Rajiv ( VA Indianapolis , INDIANAPOLIS , Indiana , United States )
- Lay-flurrie, James ( Bayer plc , Reading , United Kingdom )
- Brinker, Meike ( Bayer AG , Berlin , Germany )
- Neuen, Brendon ( George Institute for Global Health , Newtown , New South Wales , Australia )
- Amarante, Flaviana ( Bayer AG , Sao Paulo SP , Brazil )
- Hofmeister, Lucas ( Bayer AG , Berlin , Germany )
- Mcmurray, John ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Vaduganathan, Muthiah ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Filippatos, Gerasimos ( NKUA , Chaidari , Greece )
- Desai, Akshay ( BRIGHAM WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Jhund, Pardeep ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
- Henderson, Alasdair David ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
- Lam, Carolyn ( NATIONAL HEART CENTRE SINGAPORE , Singapore , Singapore )
- Senni, Michele ( ASST PAPA GIOVANNI XXIII , BERGAMO , Italy )
Meeting Info:
Session Info:
Heart Failure in CKM Syndrome: Prevention, Management and Outcomes
Monday, 11/10/2025 , 01:45PM - 02:55PM
Moderated Digital Poster Session
More abstracts on this topic:
Satish Vikyath, Pargaonkar Sumant, Slipczuk Leandro, Schenone Aldo, Maliha Maisha, Chi Kuan Yu, Sunil Kumar Sriram, Borkowski Pawel, Vyas Rhea, Rodriguez Szaszdi David Jose Javier, Kharawala Amrin, Seo Jiyoung
A Novel Thrombolytic with Anti-inflammatory Properties (JX10) Improves Neurological Outcomes in Acute Lacunar Infarct up to 12 hours After OnsetChen Edmond, Niizuma Kuniyasu, Nitika Fnu, Hasumi Keiji, Tominaga Teiji, Nishimura Naoko, Zhang Shenglin
More abstracts from these authors:
Foa' Alberto, De Sanctis Yoriko, Lam Carolyn, Senni Michele, Shah Sanjiv, Voors Adriaan, Zannad Faiez, Rossing Peter, Ruilope Luis, Anker Stefan, Pitt Bertram, Pabon Maria, Agarwal Rajiv, Mcmurray John, Solomon Scott, Vaduganathan Muthiah, Desai Akshay, Filippatos Gerasimos, Claggett Brian, Jhund Pardeep, Henderson Alasdair David, Brinker Meike, Lage Andrea, Hofmeister Lucas
Major Adverse Cardiovascular Events across the Spectrum of Cardio-Kidney-Metabolic Syndrome: A FINE-HEART Pooled AnalysisSiqueira Sara, Brinker Meike, Lay-flurrie James, Rohwedder Katja, Lam Carolyn, Senni Michele, Shah Sanjiv, Voors Adriaan, Zannad Faiez, Rossing Peter, Ruilope Luis, Pabon Maria, Anker Stefan, Pitt Bertram, Agarwal Rajiv, Mcmurray John, Solomon Scott, Vaduganathan Muthiah, Makuvire Tracy, Claggett Brian, Filippatos Gerasimos, Chatur Safia, Desai Akshay, Jhund Pardeep, Henderson Alasdair David