Final ID: MP2514
The HFpEF-ABA Algorithm and Finerenone in Chronic Kidney Disease with Type 2 Diabetes: A FINE-HEART Analysis
Purpose: To ascertain whether the HFpEF-ABA model 1) distinguishes between patients with and without HF 2) is associated with adverse cardiovascular(CV) outcomes in patients without HF and 3) modifies the efficacy of finerenone.
Methods: In this participant-level pooled analysis from FINE-HEART including 3 phase-3 trials (FINEARTS-HF and FIDELIO-DKD/FIGARO-DKD (FIDELITY)) HFpEF-ABA score (based on age, body mass index, and atrial fibrillation status) was calculated at baseline. First, the performance for HF identification in the overall cohort was assessed. Second, clinical outcomes and treatment effects of finerenone versus placebo were evaluated according to continuous and categorical (low/intermediate<75%; high≥75%) HFpEF-ABA score among FIDELITY participants without HF.
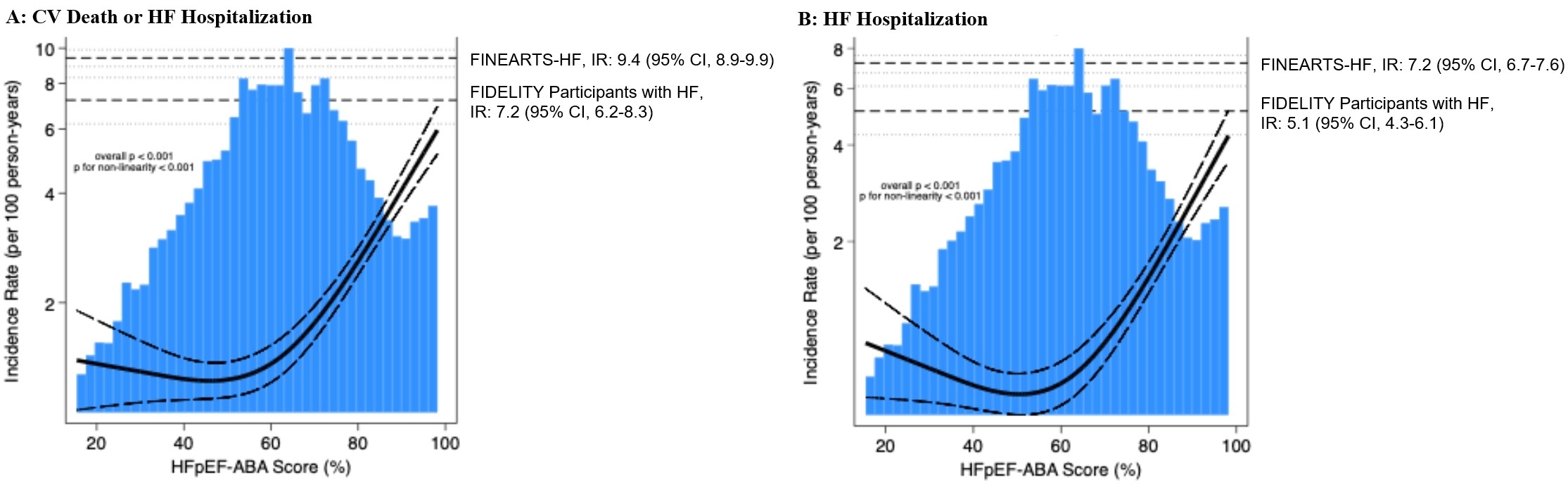
Results: Among 18,991 FINE-HEART participants, 18,943(99.7%) had a calculable HFpEF-ABA score at baseline (mean age 67±10years; 35% female; 37% with HF). The HFpEF-ABA model had moderate performance for discriminating participants with vs. without HF (Cstatistic 0.73). Among FIDELITY patients without HF (n=11,950), those with a high (28%) vs. low/intermediate (72%) HFpEF-ABA score had a higher rate of CV death or incident HF hospitalization (HR 2.20; 95%CI, 1.89-2.57;P<0.001) and incident HF hospitalization (HR 2.58; 95%CI, 2.12-3.14;P<0.001). However, the CV event rates were lower compared with established HF, even among participants with HFpEF-ABA scores >90%(Figure 1). Finerenone reduced CV death or incident HF hospitalization irrespective of baseline HFpEF-ABA score(Figure 2), with greater absolute benefits among those with a high (absolute rate reduction[ARR] 0.8 per 100 person-years (py)) vs. low/intermediate (ARR 0.2 per 100 py) HFpEF-ABA score. Serious adverse events were less common with finerenone vs. placebo in both HFpEF-ABA score categories.
Conclusions: The HFpEF-ABA model identified patients with CKD and T2D with higher risk of incident HF, but only select individuals with the highest scores experienced clinical events commensurate with those with established HF. Finerenone consistently reduced CV events across a broad HFpEF-ABA score spectrum.
- Inciardi, Riccardo ( BWH , Brescia , Italy )
- Scalise, Andrea ( Bayer AG , Berlin , Germany )
- Lam, Carolyn ( NATIONAL HEART CENTRE SINGAPORE , Singapore , Singapore )
- Senni, Michele ( ASST PAPA GIOVANNI XXIII , Bergamo , Italy )
- Shah, Sanjiv ( NORTHWESTERN UNIVERSITY , Chicago , Illinois , United States )
- Voors, Adriaan ( UNIVERSITY MEDICAL CENTER GRONINGEN , Gronien , Netherlands )
- Zannad, Faiez ( CVCT and Universite de Lorraine , Paris , France )
- Rossing, Peter ( Steno Diabetes Center Copenhagen , Gentofte , Denmark )
- Ruilope, Luis ( Hospital 12 de Octubre , Madrid , Spain )
- Anker, Stefan ( charite , Berlin , Germany )
- Pitt, Bertram ( UNIVERITY HOSPITAL , Ann Arbor , Michigan , United States )
- Ostrominski, John ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Agarwal, Rajiv ( INDIANA UNIVERSITY SCHOOL MEDICINE , Indianapolis , Indiana , United States )
- Mcmurray, John ( BHF CARDIOVASCULAR RESEARCH CENTRE , Glasgow , United Kingdom )
- Solomon, Scott ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Vaduganathan, Muthiah ( Brigham and Womens Hospital , Boston , Massachusetts , United States )
- Claggett, Brian ( Brigham and Women's Hospital , Boston , Massachusetts , United States )
- Desai, Akshay ( BRIGHAM WOMENS HOSPITAL , Boston , Massachusetts , United States )
- Jhund, Pardeep ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
- Henderson, Alasdair David ( UNIVERSITY OF GLASGOW , Glasgow , United Kingdom )
- Brinker, Meike ( Bayer AG , Berlin , Germany )
- Lay-flurrie, James ( Bayer AG , Berlin , Germany )
- Glasauer, Andrea ( Bayer AG , Berlin , Germany )
Meeting Info:
Session Info:
(Non-) Roid Rage: Novel Insights Into Non-Steroidal MRAs for HF
Monday, 11/10/2025 , 12:15PM - 01:25PM
Moderated Digital Poster Session
More abstracts on this topic:
Zhou Di, Xin Li, Liu Zhihong, Lu Minjie
Abrupt cardiac rupture of the patient with ATTR amyloidosisTagata Kento, Yutaro Nomoto, Tao Koji, Kataoka Tetsuro, Ohishi Mitsuru
More abstracts from these authors:
Ostrominski John, Shah Sanjiv, Voors Adriaan, Zannad Faiez, Rossing Peter, Ruilope Luis, Anker Stefan, Pitt Bertram, Agarwal Rajiv, Lay-flurrie James, Brinker Meike, Neuen Brendon, Amarante Flaviana, Hofmeister Lucas, Mcmurray John, Solomon Scott, Vaduganathan Muthiah, Claggett Brian, Filippatos Gerasimos, Desai Akshay, Jhund Pardeep, Henderson Alasdair David, Lam Carolyn, Senni Michele
Efficacy of finerenone in patients with heart failure and mildly reduced or preserved ejection fraction: A prespecified analysis of heart rate in the FINEARTS-HF trialChimura Misato, Senni Michele, Zannad Faiez, Pitt Bertram, Vaduganathan Muthiah, Solomon Scott, Mcmurray John, Jhund Pardeep, Henderson Alasdair David, Claggett Brian, Desai Akshay, Lay-flurrie James, Scalise Andrea, Rohwedder Katja, Lam Carolyn