Final ID: MP2733
Mitochondrial Transfer between Stromal Cells and Macrophages in Atherosclerotic Plaques Regulates Macrophage Phenotype Conversion toward the M2 Subtype
Abstract Body (Do not enter title and authors here):
Background:
Intercellular mitochondrial transfer has been documented to modulate cellular function in multiple disease contexts. Nevertheless, its occurrence and pathophysiological implications within atherosclerotic plaques remain elusive.
Research Questions:
What is the prevalence and functional significance of mitochondrial transfer within atherosclerotic plaques?
Methods:
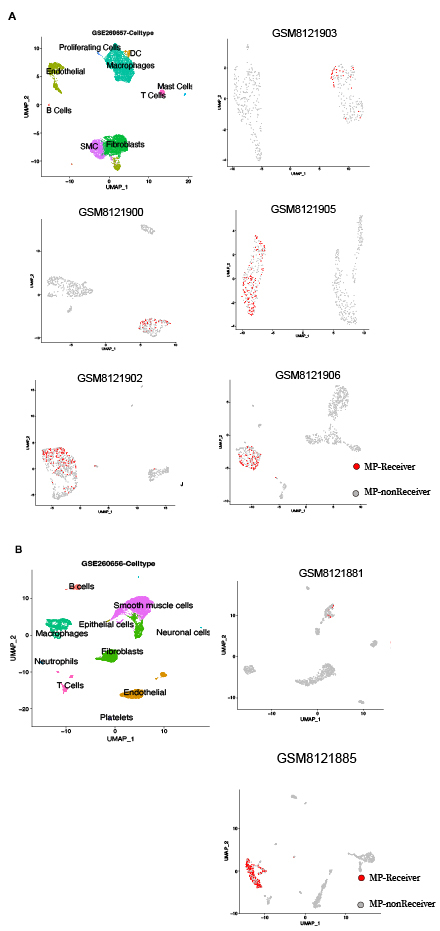
Coronary artery specimens were procured from heart transplant recipients. Pathological staging was determined through histological assessment by certified pathologists. Single-cell RNA sequencing (10X Genomics) was performed on dissociated cells, followed by cell type annotation. Mitochondrial transfer were predicted via the MERCI algorithm (positive threshold: > max of 1,000 permutations). MERCI-mtSNP identified cell type-specific mitochondrial SNP enrichments and deconvoluted mitochondrial genome coverage profiles from scRNA data. Validation cohorts included public human (GSE260657) and murine (GSE260656) atherosclerotic plaque scRNA-seq datasets. Differential expression analysis compared mitochondrial-receiving macrophages (MP-Receivers) versus non-receiving counterparts (MP-nonReceivers), with Gene Ontology (GO) enrichment elucidating associated biological pathways. Functional alterations in MP-Receivers were assessed through subcluster analysis.
Results:
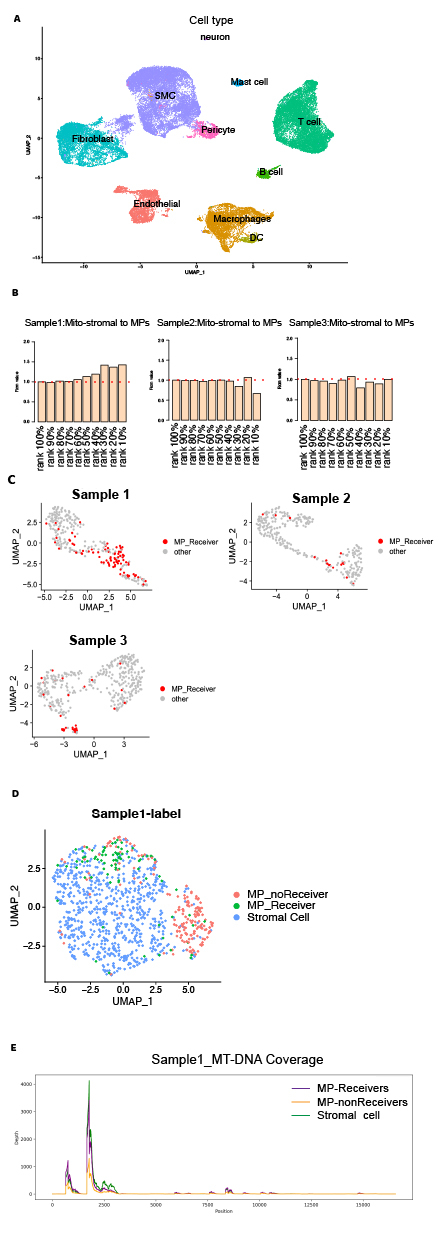
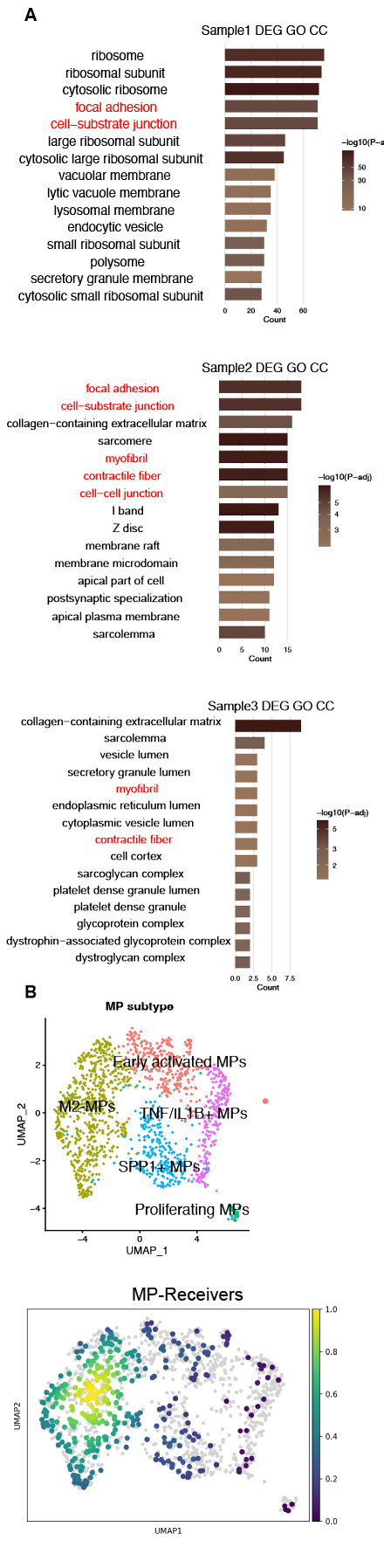
Single-cell RNA sequencing of 34,791 cells from 7 human coronary arteries identified 10 distinct cell types. MERCI analysis demonstrated mitochondrial transfer from stromal cells (endothelial/smooth muscle/fibroblasts) to macrophages in 42% (3/7) of specimens. Algorithm-predicted MP-Receivers exhibited spatial clustering in uniform manifold approximation and projection (UMAP) embeddings and displayed transitional mitochondrial gene expression profiles bridging stromal cells and MP-nonReceivers. These findings were conserved in external human and murine atherosclerotic scRNA-seq datasets. Differential expression analysis implicated tunneling nanotubes in mitochondrial trafficking pathways, while macrophage subcluster analysis revealed predominant localization of MP-Receivers within M2-polarized subsets.
Conclusion:
Mitochondrial transfer between cells occurs within the atherosclerotic plaque microenvironment, with a significant transfer of mitochondria from stromal cells to macrophages. Following mitochondrial transferred, macrophages may undergo phenotypic conversion, exhibiting a tendency toward the M2 subtype.
Background:
Intercellular mitochondrial transfer has been documented to modulate cellular function in multiple disease contexts. Nevertheless, its occurrence and pathophysiological implications within atherosclerotic plaques remain elusive.
Research Questions:
What is the prevalence and functional significance of mitochondrial transfer within atherosclerotic plaques?
Methods:
Coronary artery specimens were procured from heart transplant recipients. Pathological staging was determined through histological assessment by certified pathologists. Single-cell RNA sequencing (10X Genomics) was performed on dissociated cells, followed by cell type annotation. Mitochondrial transfer were predicted via the MERCI algorithm (positive threshold: > max of 1,000 permutations). MERCI-mtSNP identified cell type-specific mitochondrial SNP enrichments and deconvoluted mitochondrial genome coverage profiles from scRNA data. Validation cohorts included public human (GSE260657) and murine (GSE260656) atherosclerotic plaque scRNA-seq datasets. Differential expression analysis compared mitochondrial-receiving macrophages (MP-Receivers) versus non-receiving counterparts (MP-nonReceivers), with Gene Ontology (GO) enrichment elucidating associated biological pathways. Functional alterations in MP-Receivers were assessed through subcluster analysis.
Results:
Single-cell RNA sequencing of 34,791 cells from 7 human coronary arteries identified 10 distinct cell types. MERCI analysis demonstrated mitochondrial transfer from stromal cells (endothelial/smooth muscle/fibroblasts) to macrophages in 42% (3/7) of specimens. Algorithm-predicted MP-Receivers exhibited spatial clustering in uniform manifold approximation and projection (UMAP) embeddings and displayed transitional mitochondrial gene expression profiles bridging stromal cells and MP-nonReceivers. These findings were conserved in external human and murine atherosclerotic scRNA-seq datasets. Differential expression analysis implicated tunneling nanotubes in mitochondrial trafficking pathways, while macrophage subcluster analysis revealed predominant localization of MP-Receivers within M2-polarized subsets.
Conclusion:
Mitochondrial transfer between cells occurs within the atherosclerotic plaque microenvironment, with a significant transfer of mitochondria from stromal cells to macrophages. Following mitochondrial transferred, macrophages may undergo phenotypic conversion, exhibiting a tendency toward the M2 subtype.
More abstracts on this topic:
A Novel H2 Relaxin B-Chain-Only Peptide Variant B7-33 Improves The Pathophysiology Of Placental Ischemia In The Reduced Uterine Perfusion Pressure Rat Model Of Preeclampsia
Pantho Ahmed F, Hossain Mohammed, Uddin Mohammad, Amaral Lorena, Campbell Nathan, Afroze Syeda, Vora Niraj, Kuehl Thomas, Lindheim Steven, Lamarca Babbette, Bathgate Ross
Acute loss of PGC1α in adult cardiomyocytes reduces ischemia-reperfusion injuryHe Lihao, Young Martin E, Rowe Glenn, Prabhu Sumanth, Sethu Palaniappan, Xie Min, Chen Yunxi, Chu Yuxin, Hua Yutao, Cai Junyan, He Jin, Benavides Gloria, Darley-usmar Victor, Ballinger Scott