Final ID: MP568
Multimodal AI Signatures of Cardiac Remodeling Independently Predict Worse Aortic Stenosis Prognosis
Abstract Body (Do not enter title and authors here): Introduction
Aortic stenosis (AS) follows a progressive course, with delays in diagnosis linked to worse outcomes. Traditional AS categorization relies on functional Doppler parameters, which may not adequately define structural and electrophysiological remodeling linked to a higher risk of progression and adverse outcomes.
Hypothesis
Multimodal integration of AI-enabled digital biomarkers of structural, functional, and electrophysiological remodeling may enable greater precision in the phenotyping of AS risk.
Methods
We included 61,812 individuals from the UK Biobank who prospectively underwent 12-lead electrocardiography (ECG) and cardiac magnetic resonance (CMR) imaging. We quantified 3 AI-enabled digital biomarkers of AS risk: 1) Electrophysiological signature (AI-ECG score): defined as the cosine similarity (0-1) between an individual’s ECG embedding and a typical ECG embedding for AS cases as derived from a foundation model trained across a large U.S. health system; 2) Structural signature: defined as the validated Digital AS Severity index (DASSi, 0-1), a biomarker of cardiac remodeling in AS measurable on long-axis cine-CMR clips; and 3) Flow signature: defined as the peak aortic ejection velocity on velocity-encoded CMR using DeepFlow. We examined the age/sex-adjusted, independent association of the 3 biomarkers with prevalent AS, as well as incident aortic valve replacement (AVR) and mortality (Figure 1).
Results
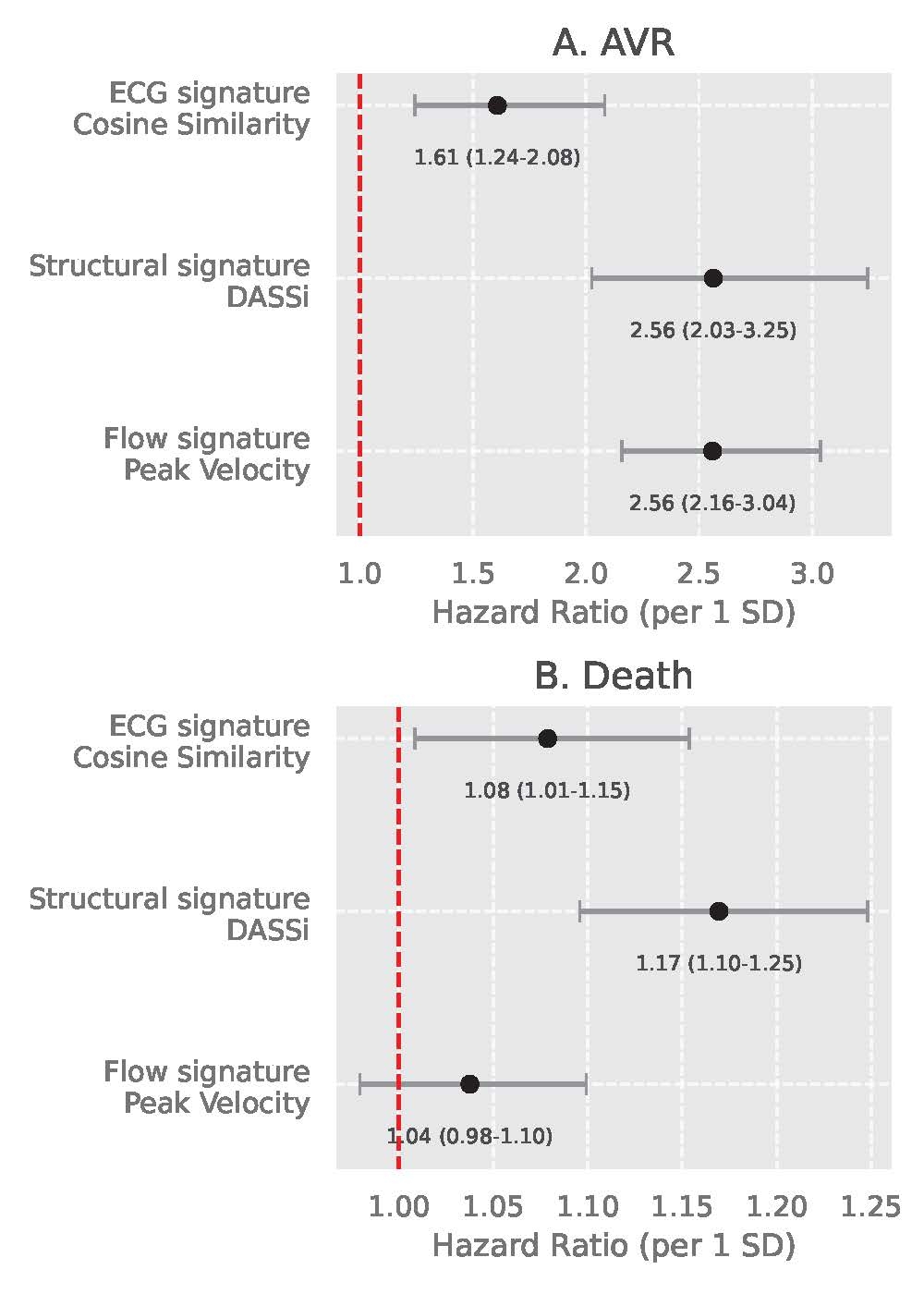
Among 61,812 individuals (mean age 66±8 years, n=32,167 [52.0%] female), 246 (0.40%) had prevalent AS at baseline. Each SD increase in the electrophysiological (AI-ECG score), structural (DASSi), and flow (peak velocity) biomarkers was associated with 1.4-, 1.8- and 2.2-fold higher odds of prevalent AS (Figure 2). Over a median follow-up of 4.6 [IQR: 1.3-6.3] years, 73 (0.12%) individuals underwent AVR and 1037 (1.7%) died. All 3 biomarkers independently predicted AS progression based on incident AVR (per 1 SD: 2.56 [95%CI: 2.16-3.04], 2.56 [2.03-3.25] and 1.61 [1.24-2.08], respectively) (Figure 3a). AI-ECG score (HR 1.08 [95%CI: 1.01-1.15]) and DASSi (1.17 [1.10-1.25]), but not peak velocity (1.04 [0.98-1.10]) were associated with mortality (Figure 3b).
Conclusion
We propose an AI-enabled multimodal approach using electrophysiological, structural and flow-related signatures to enable precision phenotyping of future AS risk. The scalability of AI-ECG and DASSi to point-of-care devices may define a novel paradigm for early AS monitoring.
Aortic stenosis (AS) follows a progressive course, with delays in diagnosis linked to worse outcomes. Traditional AS categorization relies on functional Doppler parameters, which may not adequately define structural and electrophysiological remodeling linked to a higher risk of progression and adverse outcomes.
Hypothesis
Multimodal integration of AI-enabled digital biomarkers of structural, functional, and electrophysiological remodeling may enable greater precision in the phenotyping of AS risk.
Methods
We included 61,812 individuals from the UK Biobank who prospectively underwent 12-lead electrocardiography (ECG) and cardiac magnetic resonance (CMR) imaging. We quantified 3 AI-enabled digital biomarkers of AS risk: 1) Electrophysiological signature (AI-ECG score): defined as the cosine similarity (0-1) between an individual’s ECG embedding and a typical ECG embedding for AS cases as derived from a foundation model trained across a large U.S. health system; 2) Structural signature: defined as the validated Digital AS Severity index (DASSi, 0-1), a biomarker of cardiac remodeling in AS measurable on long-axis cine-CMR clips; and 3) Flow signature: defined as the peak aortic ejection velocity on velocity-encoded CMR using DeepFlow. We examined the age/sex-adjusted, independent association of the 3 biomarkers with prevalent AS, as well as incident aortic valve replacement (AVR) and mortality (Figure 1).
Results
Among 61,812 individuals (mean age 66±8 years, n=32,167 [52.0%] female), 246 (0.40%) had prevalent AS at baseline. Each SD increase in the electrophysiological (AI-ECG score), structural (DASSi), and flow (peak velocity) biomarkers was associated with 1.4-, 1.8- and 2.2-fold higher odds of prevalent AS (Figure 2). Over a median follow-up of 4.6 [IQR: 1.3-6.3] years, 73 (0.12%) individuals underwent AVR and 1037 (1.7%) died. All 3 biomarkers independently predicted AS progression based on incident AVR (per 1 SD: 2.56 [95%CI: 2.16-3.04], 2.56 [2.03-3.25] and 1.61 [1.24-2.08], respectively) (Figure 3a). AI-ECG score (HR 1.08 [95%CI: 1.01-1.15]) and DASSi (1.17 [1.10-1.25]), but not peak velocity (1.04 [0.98-1.10]) were associated with mortality (Figure 3b).
Conclusion
We propose an AI-enabled multimodal approach using electrophysiological, structural and flow-related signatures to enable precision phenotyping of future AS risk. The scalability of AI-ECG and DASSi to point-of-care devices may define a novel paradigm for early AS monitoring.
More abstracts on this topic:
A Meta-Analysis Comparing Same-Day Discharge to Later-Day Discharge in Transcatheter Aortic Valve Replacement
Jain Hritvik, Passey Siddhant, Jain Jyoti, Goyal Aman, Wasir Amanpreet, Ahmed Mushood, Patel Nandan, Yadav Ashish, Shah Janhvi, Mehta Aryan
A Cross-scale Causal Machine Learning Framework Pinpoints Mgl2+ Macrophage Orchestrators of Balanced Arterial GrowthHan Jonghyeuk, Kong Dasom, Schwarz Erica, Takaesu Felipe, Humphrey Jay, Park Hyun-ji, Davis Michael E