Final ID: MP1367
Spatial- and attenuation-based epicardial adipose tissue features on CT calcium-score exams predict ASCVD events in immune-mediated inflammatory diseases
Abstract Body (Do not enter title and authors here): Background. Immune-mediated inflammatory diseases (IMID) such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and psoriasis increase atherosclerotic cardiovascular disease (ASCVD) risk. Epicardial adipose tissue (EAT) is biologically active fat depot that may provide prognostic information and can be opportunistically quantified on CT calcium score (CTCS) scans.
Hypothesis. Spatial- and attenuation-based EAT radiomic features differ and differentially predict ASCVD events in IMID patients versus matched controls.
Methods. Patients without known ASCVD who underwent CTCS between 2014 and 2020 in the CLARIFY Registry (NCT0407516224) were included in this analysis. A validated AI tool was used to segment EAT. Next, 215 “fat-omics” features were extracted, including spatial positioning of EAT from inferior-to-superior cardiac spanning slab quartiles (PQ), and Hounsfield unit (HU) histogram bins (20-HU increments). IMID patients were matched 2:1 on age, sex, race, hypertension, diabetes, and smoking. Incident myocardial infarction (MI)/revascularization was analyzed over a median 4-year follow-up.
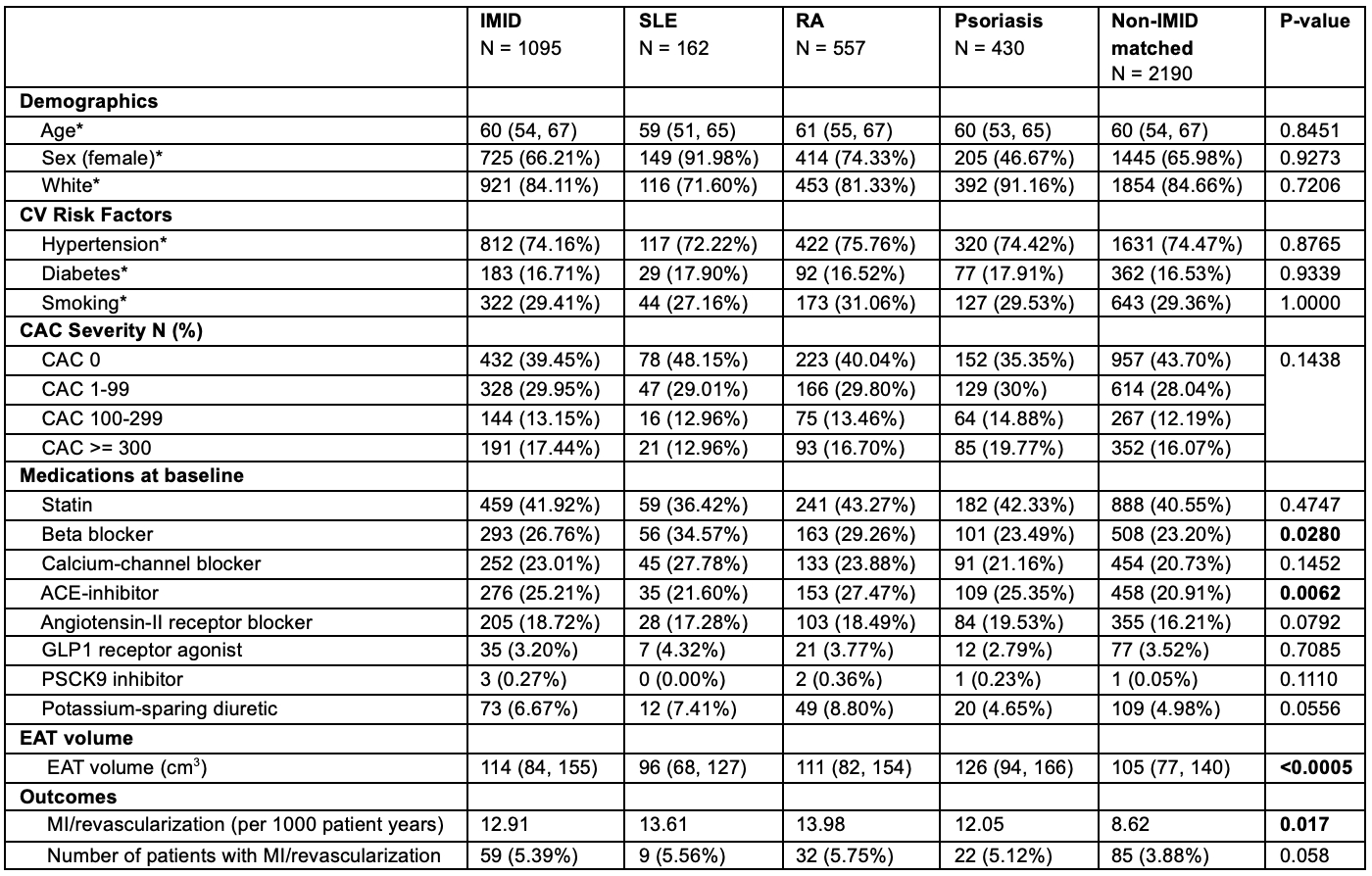
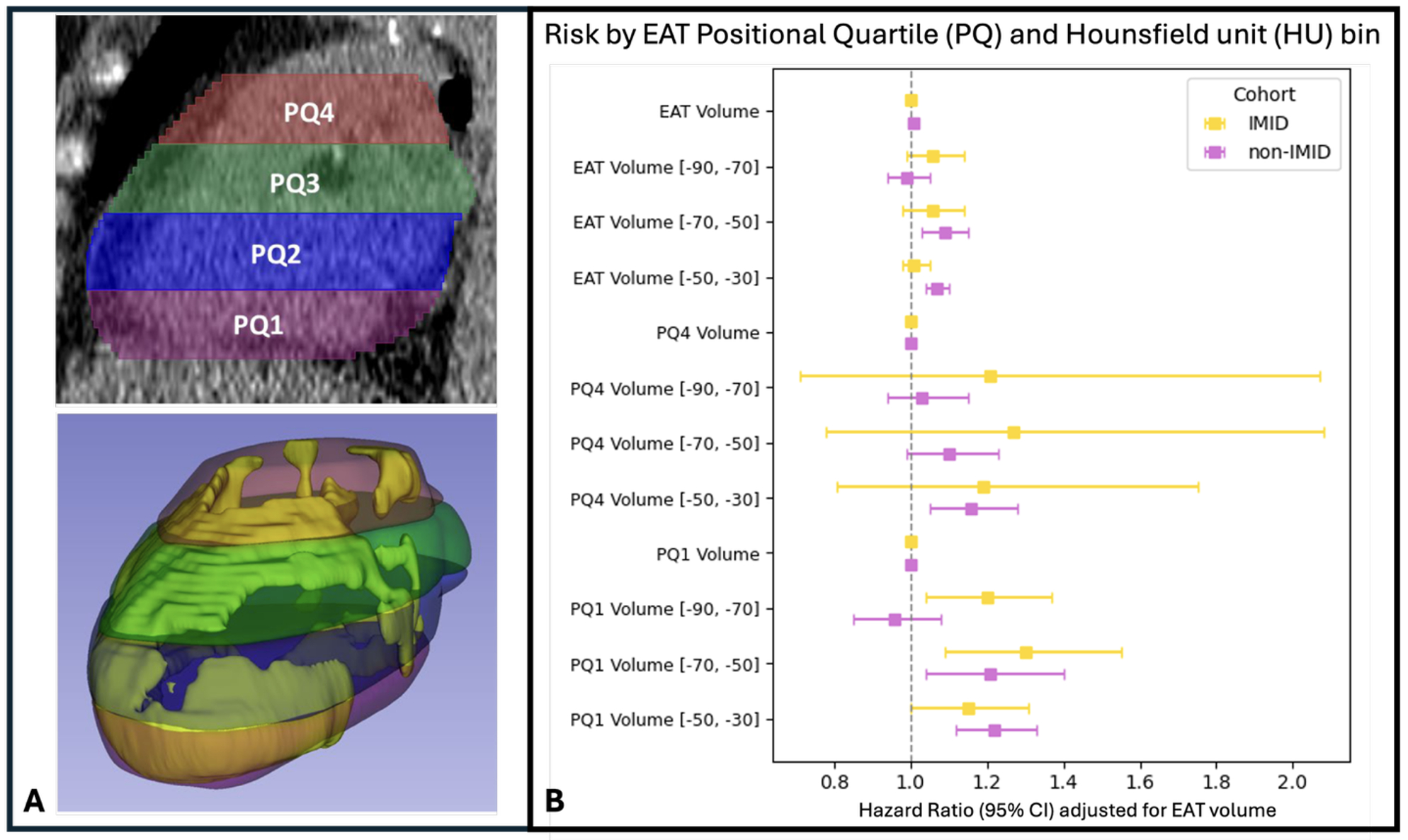
Results. IMID patients (N=1095, SLE: 162, RA: 557, Psoriasis: 430) had significantly higher EAT volume (p<0.005) and a greater incidence of MI/revascularization (12.9 vs. 8.6 per 1000 person-years) than matched controls (n = 2190). After false-discovery-rate correction, 70 features differed, driven by high-density EAT (HEAT), with HU between -90 and -30. Consistent with prior work, controls demonstrated that high-attenuation EAT (Hi-HEAT) in the superior cardiac spanning slab (PQ4, HU [-50, –30]) was associated with ASCVD events (HR 1.16 [1.05–1.28]; p=0.005). Hi-HEAT was absent in IMID patients. Instead, Lo-HEAT (HU [-90, -70], [-70, -50], [-50, -30]) in the inferior slab (PQ1) predicted events with significant HRs of 1.20 [1.04, 1.37], 1.30 [1.09–1.55], and 1.15 [1.00, 1.31] respectively after adjusting for EAT volume.
Conclusion. Spatial- and attenuation-based EAT radiomics reveal a distinct inferio-superior spatially segregate phenotype (Lo-HEAT) that drives ASCVD risk in IMID patients, suggesting differential EAT relationships. Lo-HEAT might reflect more systemic EAT inflammation as opposed to focal coronary inflammation (Hi-HEAT). Future studies should prospectively validate the Lo-HEAT phenotype and test whether targeted anti-inflammatory therapies can mitigate ASCVD risk in IMID patients.
Hypothesis. Spatial- and attenuation-based EAT radiomic features differ and differentially predict ASCVD events in IMID patients versus matched controls.
Methods. Patients without known ASCVD who underwent CTCS between 2014 and 2020 in the CLARIFY Registry (NCT0407516224) were included in this analysis. A validated AI tool was used to segment EAT. Next, 215 “fat-omics” features were extracted, including spatial positioning of EAT from inferior-to-superior cardiac spanning slab quartiles (PQ), and Hounsfield unit (HU) histogram bins (20-HU increments). IMID patients were matched 2:1 on age, sex, race, hypertension, diabetes, and smoking. Incident myocardial infarction (MI)/revascularization was analyzed over a median 4-year follow-up.
Results. IMID patients (N=1095, SLE: 162, RA: 557, Psoriasis: 430) had significantly higher EAT volume (p<0.005) and a greater incidence of MI/revascularization (12.9 vs. 8.6 per 1000 person-years) than matched controls (n = 2190). After false-discovery-rate correction, 70 features differed, driven by high-density EAT (HEAT), with HU between -90 and -30. Consistent with prior work, controls demonstrated that high-attenuation EAT (Hi-HEAT) in the superior cardiac spanning slab (PQ4, HU [-50, –30]) was associated with ASCVD events (HR 1.16 [1.05–1.28]; p=0.005). Hi-HEAT was absent in IMID patients. Instead, Lo-HEAT (HU [-90, -70], [-70, -50], [-50, -30]) in the inferior slab (PQ1) predicted events with significant HRs of 1.20 [1.04, 1.37], 1.30 [1.09–1.55], and 1.15 [1.00, 1.31] respectively after adjusting for EAT volume.
Conclusion. Spatial- and attenuation-based EAT radiomics reveal a distinct inferio-superior spatially segregate phenotype (Lo-HEAT) that drives ASCVD risk in IMID patients, suggesting differential EAT relationships. Lo-HEAT might reflect more systemic EAT inflammation as opposed to focal coronary inflammation (Hi-HEAT). Future studies should prospectively validate the Lo-HEAT phenotype and test whether targeted anti-inflammatory therapies can mitigate ASCVD risk in IMID patients.
More abstracts on this topic:
A Site-by-Site Comparison of CT Attenuation, Effective Atomic Numbers, and Electron Densities of Focal Pericoronary Adipose Tissue and Its Relationship to Adjacent Coronary Plaques on Contrast Enhanced Spectral CT
Kaneko Aya, Sakaguchi Yamato, Funabashi Nobusada
A Cross-scale Causal Machine Learning Framework Pinpoints Mgl2+ Macrophage Orchestrators of Balanced Arterial GrowthHan Jonghyeuk, Kong Dasom, Schwarz Erica, Takaesu Felipe, Humphrey Jay, Park Hyun-ji, Davis Michael E