Final ID: Sa2047
Survodutide for the Treatment of Obesity: Baseline characteristics of the SYNCHRONIZE Cardiovascular Outcomes Trial
Abstract Body (Do not enter title and authors here): Background: Dual agonism of glucagon and glucagon-like peptide-1 (GLP-1) receptors is effective in reducing body weight and fat mass, but its cardiovascular (CV) effects are unknown.
Aims: The primary objective of the SYNCHRONIZE-CV outcomes trial (CVOT) is to test non-inferiority of survodutide compared with placebo for time-to-first occurrence of any of the adjudicated components of the primary composite endpoint of 5-point major adverse CV events, defined as CV death, non-fatal stroke, non-fatal myocardial infarction, ischemia-related coronary revascularization, or heart failure events.
Methods: We describe the baseline characteristics of participants in the SYNCHRONIZE-CVOT, a phase 3, randomized, double-blind, parallel-group, event-driven, CV safety study of survodutide, a dual glucagon and GLP-1 receptor agonist, compared with placebo in adults with a body mass index ≥27 kg/m2 and established CV or chronic kidney disease (CKD), and/or at least 2 obesity-related complications or risk factors for CV disease (CVD). Participants were randomized 1:1:1 to once-weekly subcutaneous injections of survodutide (up-titrated to 3.6 or 6.0 mg) or placebo, in addition to standard lifestyle-based care.
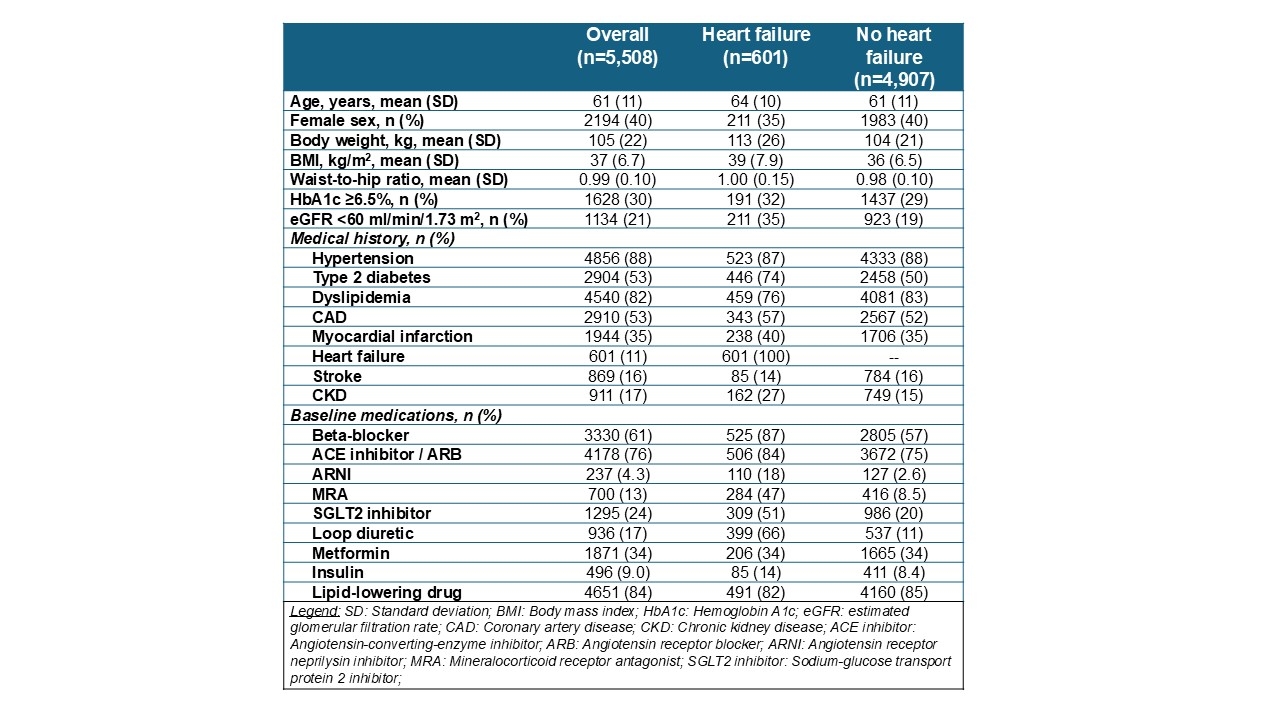
Results: Overall, 5,508 participants were randomized to and treated with survodutide or placebo across 524 sites in 34 countries (mean age 61 years, 40% women, mean body mass index 37 kg/m2, mean estimated glomerular filtration rate 77 mL/min/1.73m2; Table). At baseline, common CV risk factors included hypertension (88%), dyslipidemia (82%) and type 2 diabetes (53%). Established CVD included coronary artery disease (53%), prior myocardial infarction (35%), prior stroke (16%) and heart failure (11%); and 17% of participants had CKD. SYNCHRONIZE-CVOT enrolled a pre-specified subgroup of individuals with prevalent heart failure. Among these 601 participants with heart failure (NYHA II-III at screening), median NT-proBNP was 424 pg/ml and mean Kansas City Cardiomyopathy Questionnaire Total Symptom Score was 71. At baseline, more than half of participants with heart failure were treated with SGLT2 inhibitors and 66% with loop diuretics.
Conclusions: SYNCHRONIZE-CVOT enrolled people who were overweight or obese across a broad spectrum of CVD and CKD risk categories. SYNCHRONIZE-CVOT is the first randomized, placebo-controlled, phase 3 trial that will determine the CV safety of survodutide in people with overweight/obesity and increased CV risk.
Aims: The primary objective of the SYNCHRONIZE-CV outcomes trial (CVOT) is to test non-inferiority of survodutide compared with placebo for time-to-first occurrence of any of the adjudicated components of the primary composite endpoint of 5-point major adverse CV events, defined as CV death, non-fatal stroke, non-fatal myocardial infarction, ischemia-related coronary revascularization, or heart failure events.
Methods: We describe the baseline characteristics of participants in the SYNCHRONIZE-CVOT, a phase 3, randomized, double-blind, parallel-group, event-driven, CV safety study of survodutide, a dual glucagon and GLP-1 receptor agonist, compared with placebo in adults with a body mass index ≥27 kg/m2 and established CV or chronic kidney disease (CKD), and/or at least 2 obesity-related complications or risk factors for CV disease (CVD). Participants were randomized 1:1:1 to once-weekly subcutaneous injections of survodutide (up-titrated to 3.6 or 6.0 mg) or placebo, in addition to standard lifestyle-based care.
Results: Overall, 5,508 participants were randomized to and treated with survodutide or placebo across 524 sites in 34 countries (mean age 61 years, 40% women, mean body mass index 37 kg/m2, mean estimated glomerular filtration rate 77 mL/min/1.73m2; Table). At baseline, common CV risk factors included hypertension (88%), dyslipidemia (82%) and type 2 diabetes (53%). Established CVD included coronary artery disease (53%), prior myocardial infarction (35%), prior stroke (16%) and heart failure (11%); and 17% of participants had CKD. SYNCHRONIZE-CVOT enrolled a pre-specified subgroup of individuals with prevalent heart failure. Among these 601 participants with heart failure (NYHA II-III at screening), median NT-proBNP was 424 pg/ml and mean Kansas City Cardiomyopathy Questionnaire Total Symptom Score was 71. At baseline, more than half of participants with heart failure were treated with SGLT2 inhibitors and 66% with loop diuretics.
Conclusions: SYNCHRONIZE-CVOT enrolled people who were overweight or obese across a broad spectrum of CVD and CKD risk categories. SYNCHRONIZE-CVOT is the first randomized, placebo-controlled, phase 3 trial that will determine the CV safety of survodutide in people with overweight/obesity and increased CV risk.
More abstracts on this topic:
A multi-task deep learning algorithm for detecting obstructive coronary artery disease using fundus photographs
Zeng Yong, Ding Yaodong
Adoptive Transfer of Lupus Patient PBMCs Promotes Salt-Sensitive Hypertension and Kidney Injury in Immunodeficient MiceSaleem Mohammad, Ormseth Michelle, Kirabo Annet, Ahmad Taseer, Haynes Alexandria, Albritton Claude, Arshad Suha, Kulapatana Phicharmon, Posey Olivia, Major Amy, Stein Charles