Final ID: 051
Adoptive Transfer of Lupus Patient PBMCs Promotes Salt-Sensitive Hypertension and Kidney Injury in Immunodeficient Mice
Abstract Body: Introduction: Salt sensitivity of blood pressure (SSBP) is an independent risk factor for cardiovascular disease (CVD), even in normotensive people. Systemic lupus erythematosus (SLE) is also a risk factor for CVD and hypertension. SLE and CVD are on the rise, and it is unknown if this is attributable to the increased dietary salt in the modern diet.
Hypothesis: We hypothesized that the adoptive transfer of peripheral blood mononuclear cells (PBMCs) from patients with SLE/lupus into immunodeficient mice (NSG/MHCI/MHCII-/-) would increase inflammation and SSBP after high salt feeding.
Method: In vitro studies were performed using PBMCs isolated from patients with SLE and treated with and without high salt. We humanized mice with PBMCs from patients with SLE (n=7) and controls without autoimmune disease (n=7) in animal studies. The mice were fed a 4% high-salt diet for two weeks. Blood pressure was measured with radiotelemetry, immune phenotyping was performed with flow cytometry, and vascular reactivity was assessed in mesenteric arteries using wire myography.
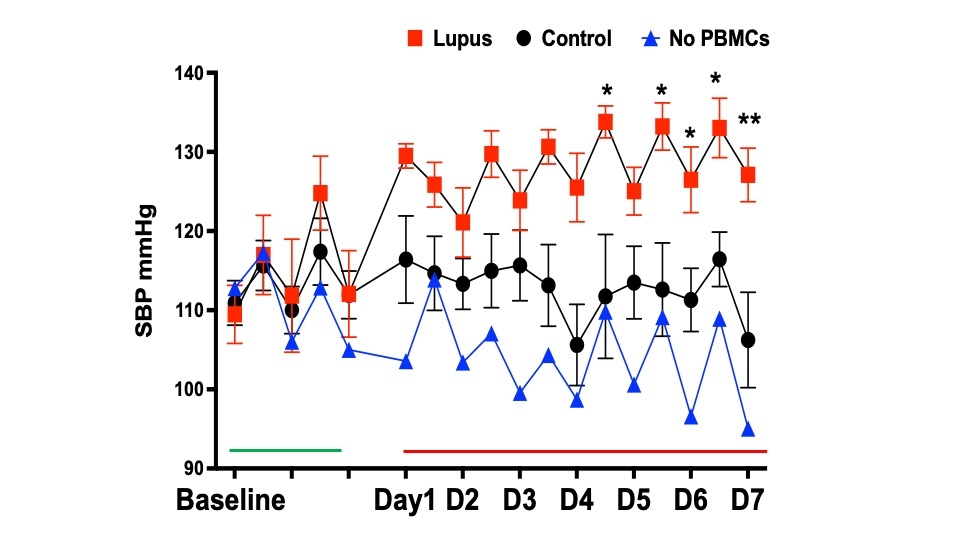
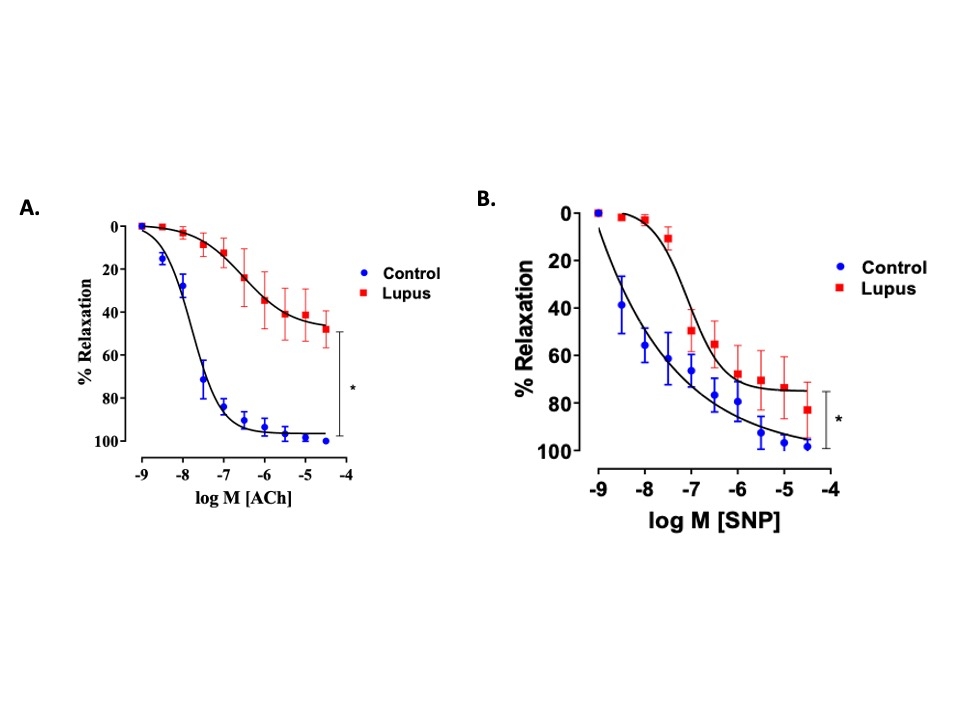
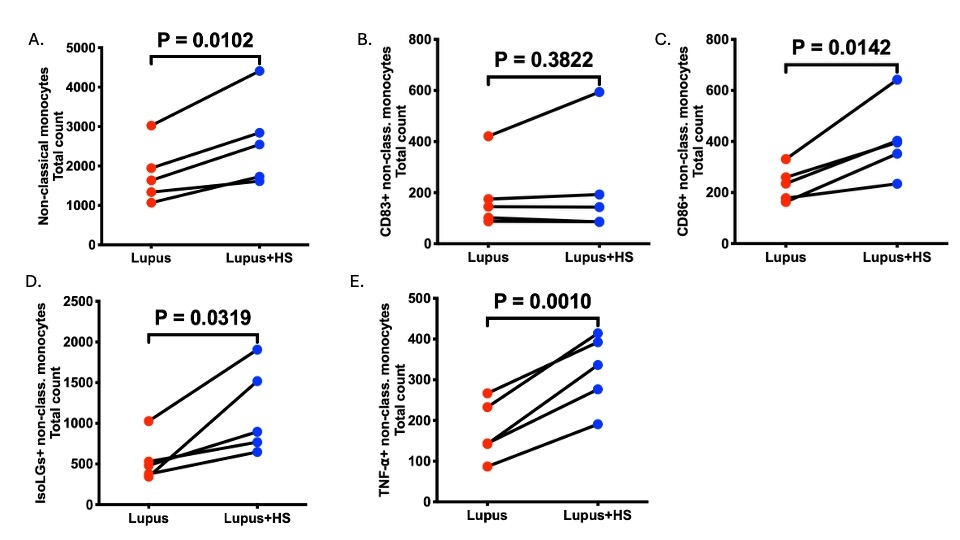
Results: A high salt diet significantly increased systolic blood pressure in lupus mice compared to the controls (133.0 vs.116.4 mmHg, p=0.001). APC infiltration (macrophages, monocytes, dendritic cells), proinflammatory markers, and oxidative stress (Isolevuglandins, Nox2) were significantly higher in the kidneys of mice receiving the PBMC from the SLE patients (lupus mice). Innate and adaptive immune cells and proinflammatory markers were significantly elevated in the kidney, spleen, and aorta. These mice also exhibited mesenteric artery vascular dysfunction (p<0.05). Lupus mice excreted more urinary albumin than the controls (25.89 vs. 17.24 µg/ml p=0.142), indicating kidney injury. High salt treatment in vitro increased intermediate and nonclassical monocytes with increased CD86, IsoLG, and TNF-alpha expression compared to normal salt-treated cells. 3D mitochondrial analysis revealed increased mitochondrial fragmentation in high salt-treated monocytes, indicating salt-induced morphological alterations.
In conclusion, our findings suggest that immune cells from SLE patients, when exposed to high salt, promote salt-sensitive hypertension (SSBP) and a proinflammatory state in the kidney, aorta, and spleen. These changes impair vascular function, alter mitochondrial morphology, and exacerbate albuminuria in immunodeficient mice.
Hypothesis: We hypothesized that the adoptive transfer of peripheral blood mononuclear cells (PBMCs) from patients with SLE/lupus into immunodeficient mice (NSG/MHCI/MHCII-/-) would increase inflammation and SSBP after high salt feeding.
Method: In vitro studies were performed using PBMCs isolated from patients with SLE and treated with and without high salt. We humanized mice with PBMCs from patients with SLE (n=7) and controls without autoimmune disease (n=7) in animal studies. The mice were fed a 4% high-salt diet for two weeks. Blood pressure was measured with radiotelemetry, immune phenotyping was performed with flow cytometry, and vascular reactivity was assessed in mesenteric arteries using wire myography.
Results: A high salt diet significantly increased systolic blood pressure in lupus mice compared to the controls (133.0 vs.116.4 mmHg, p=0.001). APC infiltration (macrophages, monocytes, dendritic cells), proinflammatory markers, and oxidative stress (Isolevuglandins, Nox2) were significantly higher in the kidneys of mice receiving the PBMC from the SLE patients (lupus mice). Innate and adaptive immune cells and proinflammatory markers were significantly elevated in the kidney, spleen, and aorta. These mice also exhibited mesenteric artery vascular dysfunction (p<0.05). Lupus mice excreted more urinary albumin than the controls (25.89 vs. 17.24 µg/ml p=0.142), indicating kidney injury. High salt treatment in vitro increased intermediate and nonclassical monocytes with increased CD86, IsoLG, and TNF-alpha expression compared to normal salt-treated cells. 3D mitochondrial analysis revealed increased mitochondrial fragmentation in high salt-treated monocytes, indicating salt-induced morphological alterations.
In conclusion, our findings suggest that immune cells from SLE patients, when exposed to high salt, promote salt-sensitive hypertension (SSBP) and a proinflammatory state in the kidney, aorta, and spleen. These changes impair vascular function, alter mitochondrial morphology, and exacerbate albuminuria in immunodeficient mice.
More abstracts on this topic:
ACTIVATION AND TARGETABILITY OF TYMP-IL-6-TF AXIS IN THE SKIN MICROENVIRONMENT IN UREMIC CALCIPHYLAXIS
Lotfollahzadeh Saran, Chitalia Vipul
Adverse effect of Autoimmune Diseases on In-hospital Cardiac Arrest MortalityPius Ruth, Markson Favour, Antia Akanimo, Odugbemi Olufemi, Nwogwugwu Enyioma, Ong Kenneth