Final ID: MP798
Safety and Tolerability of REGN5381, a Monoclonal Antibody Agonist of NPR1 in Patients With Heart Failure With Reduced Ejection Fraction
Abstract Body (Do not enter title and authors here): Introduction: Heart failure with reduced ejection fraction (HFrEF) causes high morbidity and mortality despite available therapies, indicating an unmet medical need. Activation of natriuretic peptide receptor 1 (NPR1) regulates vascular tone, lowers venous pressures, and affects natriuresis and diuresis, which may have therapeutic benefit. Recombinant natriuretic peptide infusions, though promising, were limited by short duration of effect. REGN5381, an investigational NPR1 agonist, has shown hemodynamic effects without adverse systemic hypotension along with sustained bioavailability after a single-dose in preclinical studies and a first-in-human study (NCT04506645).
Research question: How does REGN5381 impact safety, tolerability, and hemodynamic parameters in patients with HFrEF?
Methods: Patients aged 18–75 years, with New York Heart Association class II/III heart failure with left ventricular ejection fraction ≥20% and <50%, were enrolled in this phase 2a, double-blind study (NCT05353166) and received single intravenous infusions of REGN5381 at doses between 10 mg and 300 mg or placebo. Patients taking sacubitril-valsartan were excluded. Safety was monitored as treatment-emergent adverse events (TEAEs). Pulmonary capillary wedge pressure (PCWP), systemic blood pressure (BP), and biomarkers such as N-terminal pro B-type natriuretic peptide (NT-proBNP) were measured at baseline and at 2, 4, and 6 h post-infusion.
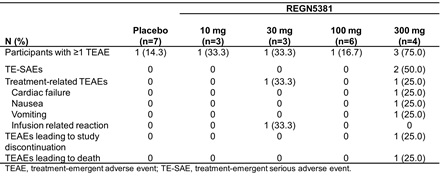
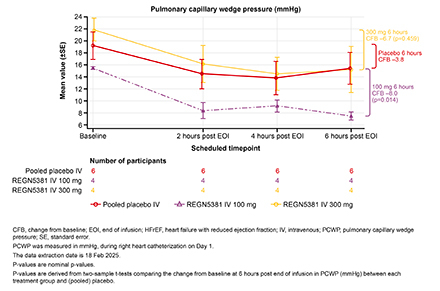
Results: Twenty-three patients received REGN5381 10 mg (n=3), 30 mg (n=3), 100 mg (n=6), or 300 mg (n=4); 7 received placebo. Compared with placebo, numeric reductions in PCWP were observed with the 100 mg and 300 mg doses at 6 h post-infusion (Fig. 1). Baseline mean (SD) systolic BP was 130.6 (17.9), 118.7 (9.2), and 123.0 (30.0) mmHg for the placebo, 100 mg, and 300 mg groups, with changes from baseline 6 h post-infusion of −1.3 (2.6), −5.7 (7.3), and −7.5 (15.6) mmHg. No apparent differences or persistent trends in NT-proBNP and systemic BP reductions among dose groups were noted. TEAEs were observed in 7 patients (Table). Two severe serious TEAEs occurred in the 300 mg group, ischemic hepatitis and cardiac failure, which was possibly related to treatment and resulted in death 10 days after study drug administration.
Conclusions: In this small phase 2 trial, REGN5381 was generally well tolerated. However, more data are needed to confirm its safety. The numeric reduction in PCWP suggests REGN5381 may aid in heart failure decongestion.
Research question: How does REGN5381 impact safety, tolerability, and hemodynamic parameters in patients with HFrEF?
Methods: Patients aged 18–75 years, with New York Heart Association class II/III heart failure with left ventricular ejection fraction ≥20% and <50%, were enrolled in this phase 2a, double-blind study (NCT05353166) and received single intravenous infusions of REGN5381 at doses between 10 mg and 300 mg or placebo. Patients taking sacubitril-valsartan were excluded. Safety was monitored as treatment-emergent adverse events (TEAEs). Pulmonary capillary wedge pressure (PCWP), systemic blood pressure (BP), and biomarkers such as N-terminal pro B-type natriuretic peptide (NT-proBNP) were measured at baseline and at 2, 4, and 6 h post-infusion.
Results: Twenty-three patients received REGN5381 10 mg (n=3), 30 mg (n=3), 100 mg (n=6), or 300 mg (n=4); 7 received placebo. Compared with placebo, numeric reductions in PCWP were observed with the 100 mg and 300 mg doses at 6 h post-infusion (Fig. 1). Baseline mean (SD) systolic BP was 130.6 (17.9), 118.7 (9.2), and 123.0 (30.0) mmHg for the placebo, 100 mg, and 300 mg groups, with changes from baseline 6 h post-infusion of −1.3 (2.6), −5.7 (7.3), and −7.5 (15.6) mmHg. No apparent differences or persistent trends in NT-proBNP and systemic BP reductions among dose groups were noted. TEAEs were observed in 7 patients (Table). Two severe serious TEAEs occurred in the 300 mg group, ischemic hepatitis and cardiac failure, which was possibly related to treatment and resulted in death 10 days after study drug administration.
Conclusions: In this small phase 2 trial, REGN5381 was generally well tolerated. However, more data are needed to confirm its safety. The numeric reduction in PCWP suggests REGN5381 may aid in heart failure decongestion.
More abstracts on this topic:
Association of natriuretic peptide concentration with lifetime risk of heart failure in adults with diabetes mellitus: a pooled cohort analysis
Segar Matthew, Busui Rodica, Wilkins John, Espeland Mark, Bertoni Alain, Bayes-genis Antoni, Pandey Ambarish, Patel Kershaw, De Lemos James, Vaduganathan Muthiah, Ballantyne Christie, Defilippi Chris, Ayers Colby, Januzzi James, Dullaart Robin
β1 Adrenergic Receptor Autoantibodies Promote Heart Failure Though Activation of Prostaglandin E2 Receptor EP1/Phosphodiesterase 4B PathwayCao Ning, Qiu Hui, Li Hongwei