Final ID: MP1478
Use of a Principled Framework to Compare Cardiovascular Outcomes from the FOURIER Trial to a FOURIER like-External Control Arm Using Real-World Data
Abstract Body (Do not enter title and authors here): Background: In open-label extension studies, concurrent control arms are not always feasible. Further Cardiovascular Outcomes Research with PCSK9 Inhibition in subjects with Elevated Risk (FOURIER) open label extension demonstrated the long-term safety of evolocumab. However, in the absence of a concurrent placebo arm, long-term treatment effect could not be estimated.
Aim: To create an external control arm (ECA) using real world data that is comparable to the FOURIER placebo arm to support estimation of long-term treatment effect.
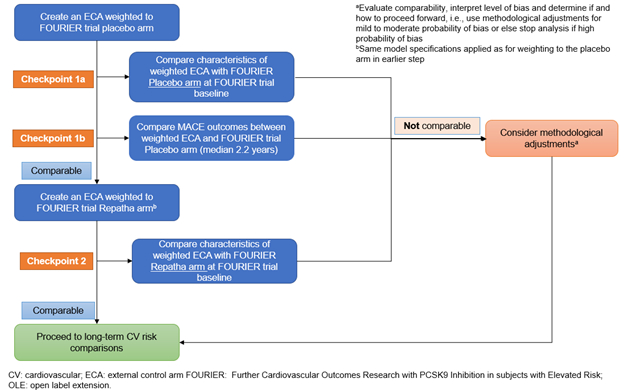
Methods: A FOURIER-like ECA was created from the UK Clinical Practice Research Datalink (CPRD) Aurum data using trial eligibility criteria and enrollment windows. The primary endpoint was a 4-point (4P) major adverse cardiovascular event (MACE) composite of myocardial infarction (MI), stroke, coronary revascularization and cardiovascular (CV) death. The key secondary endpoint was a 3-point (3P) composite of MI, stroke and CV death. A pre-specified principled approach with two checkpoints was used to evaluate the comparability of the ECA with FOURIER (Figure 1). Baseline characteristics of the ECA were compared to FOURIER placebo and evolocumab arms separately after applying inverse probability of treatment weights, using standardized mean differences (SMDs) [comparability criterion: SMD <0.15]. Additionally, all endpoint event rates were compared between FOURIER placebo arm and ECA over the FOURIER trial duration. Corresponding hazard ratios (HR) and 95% CI were calculated after applying inverse probability of censoring weights [comparability criterion: overlapping 95% CI for both event rates and HR]. Endpoints that meet both criteria could then be compared to evaluate long-term treatment effect.
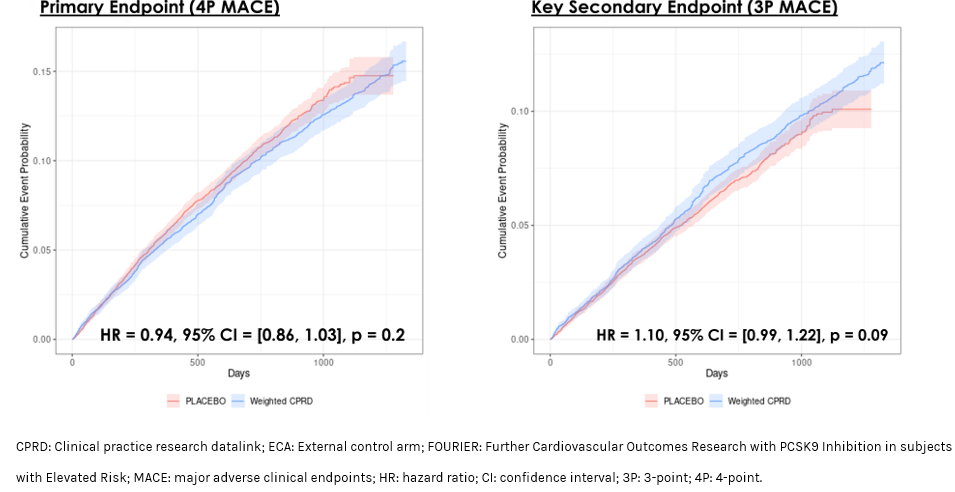
Results: In CPRD data, 56,490 patients met the FOURIER eligibility criteria. After weighting, all SMDs for baseline characteristics were <0.15 for the ECA vs. FOURIER placebo and evolocumab arms, separately, establishing baseline comparability. Over 2.2 years, the 4P MACE and 3P MACE endpoints were comparable between the FOURIER placebo arm and weighted ECA (HR for 4P MACE: 0.94, 95% CI: 0.86, 1.03; HR for 3P MACE: 1.10, 95%CI: 0.99, 1.22) (Figure 2).
Conclusion: These results suggest that a principled framework can be used to develop a trial-like cohort from real world data and may be a useful tool to potentially evaluate long-term treatment benefits of cardiovascular medicines, when appropriately analyzed and gated.
Aim: To create an external control arm (ECA) using real world data that is comparable to the FOURIER placebo arm to support estimation of long-term treatment effect.
Methods: A FOURIER-like ECA was created from the UK Clinical Practice Research Datalink (CPRD) Aurum data using trial eligibility criteria and enrollment windows. The primary endpoint was a 4-point (4P) major adverse cardiovascular event (MACE) composite of myocardial infarction (MI), stroke, coronary revascularization and cardiovascular (CV) death. The key secondary endpoint was a 3-point (3P) composite of MI, stroke and CV death. A pre-specified principled approach with two checkpoints was used to evaluate the comparability of the ECA with FOURIER (Figure 1). Baseline characteristics of the ECA were compared to FOURIER placebo and evolocumab arms separately after applying inverse probability of treatment weights, using standardized mean differences (SMDs) [comparability criterion: SMD <0.15]. Additionally, all endpoint event rates were compared between FOURIER placebo arm and ECA over the FOURIER trial duration. Corresponding hazard ratios (HR) and 95% CI were calculated after applying inverse probability of censoring weights [comparability criterion: overlapping 95% CI for both event rates and HR]. Endpoints that meet both criteria could then be compared to evaluate long-term treatment effect.
Results: In CPRD data, 56,490 patients met the FOURIER eligibility criteria. After weighting, all SMDs for baseline characteristics were <0.15 for the ECA vs. FOURIER placebo and evolocumab arms, separately, establishing baseline comparability. Over 2.2 years, the 4P MACE and 3P MACE endpoints were comparable between the FOURIER placebo arm and weighted ECA (HR for 4P MACE: 0.94, 95% CI: 0.86, 1.03; HR for 3P MACE: 1.10, 95%CI: 0.99, 1.22) (Figure 2).
Conclusion: These results suggest that a principled framework can be used to develop a trial-like cohort from real world data and may be a useful tool to potentially evaluate long-term treatment benefits of cardiovascular medicines, when appropriately analyzed and gated.
More abstracts on this topic:
Cardiologist Attitude to Lipid Control in Patients with ASCVD without Major Cardiovascular Events: Insights from the 2024 ACS EuroPath Survey
Zaman Azfar, De Caterina Raffaele, Schiele Francois, Sionis Alessandro, Catapano Alberico, Laufs Ulrich
Cholesterol Management in US Individuals with ASCVD within the Family Heart Database® during 2022/23: Current State of Care and Opportunities for ImprovementMacdougall Diane, Ferdinand Keith, Baum Seth, Sperling Laurence, Hartsuff Bonnie, Wilemon Katherine, Nissen Steven