Final ID: MP1466
Real-World Effectiveness of Evolocumab in Reducing Major Adverse Cardiovascular Events in Patients With Atherosclerotic Cardiovascular Disease
Abstract Body (Do not enter title and authors here): Background: Evolocumab significantly reduces major adverse cardiovascular events (MACE) in patients with atherosclerotic cardiovascular disease (ASCVD) in randomized controlled trials (RCT). However, evidence on its effectiveness in real-world (RW) clinical practice remains limited.
Aim: To evaluate the RW effectiveness of evolocumab in reducing MACE in patients with ASCVD.
Methods: Patients (≥ 18 years) who initiated evolocumab between 2017 and 2023 with a history of ASCVD were identified from the Komodo research database. The index date was defined as day 75 following the initial prescription of evolocumab. Patients were classified into two cohorts: the treated cohort, who initiated and remained on evolocumab, and the non-treated cohort, who initiated but discontinued the evolocumab prior to the index date. The non-treated cohort was selected to limit bias related to the initial treatment decision, leveraging the context from RCT that early discontinuation of evolocumab within the first 75 days has no known sustained clinical benefit. The primary outcome was the composite MACE of MI, stroke, and coronary revascularization. Patients were followed from the index date until the earliest of the following: occurrence of outcome, discontinuation of evolocumab (for treated cohort), reinitiation of evolocumab or other PCSK9 inhibitors (for non-treated cohort), end of database, or death. Cumulative incidence of outcome and 4-year risk ratio (RR) between treatment cohorts were calculated to evaluate the effectiveness of evolocumab, adjusting for confounders and informative censoring using inverse probability of treatment and censoring weights. Potential residual confounding was evaluated using negative controls prior to initiating the outcome analysis.
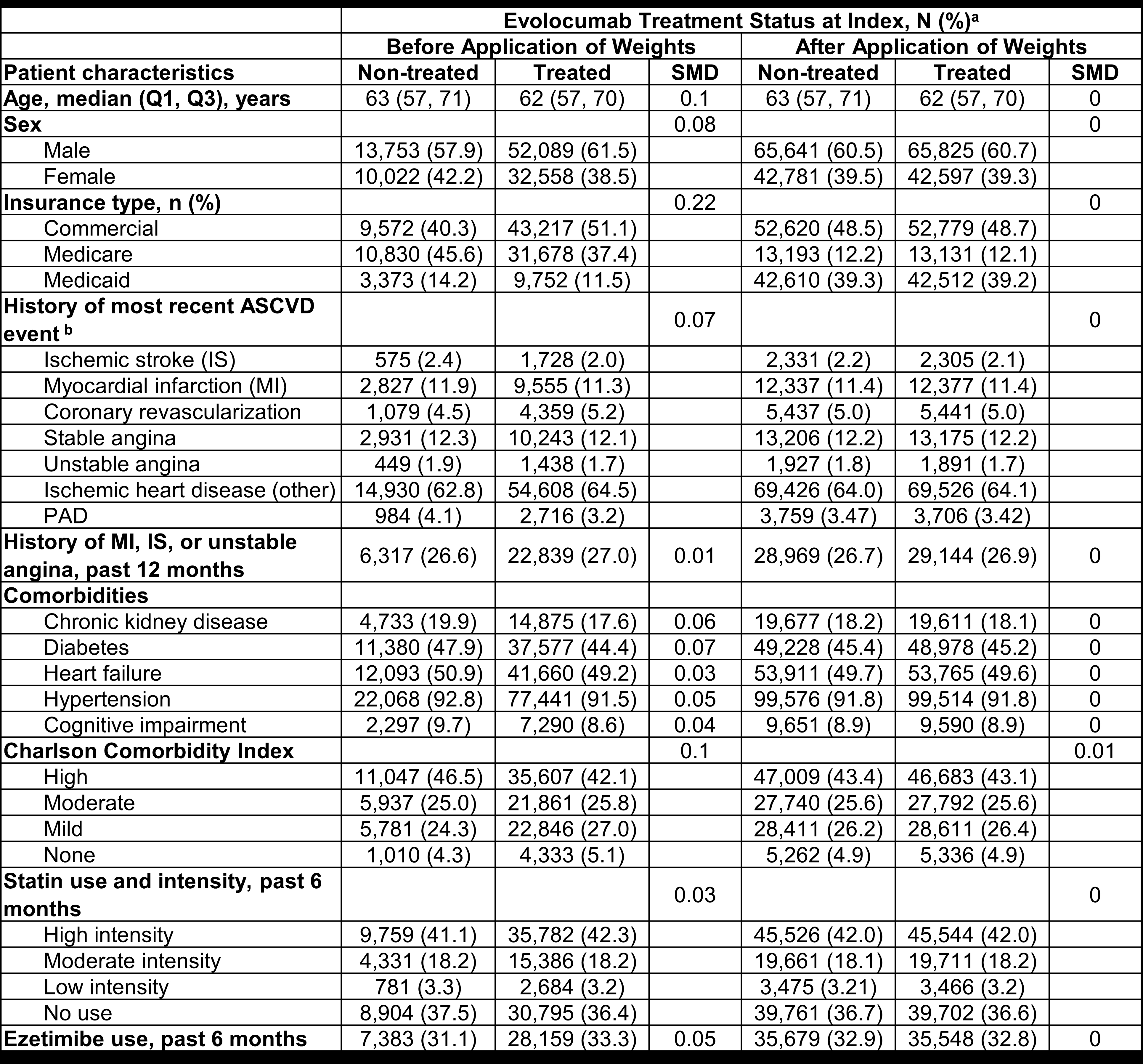
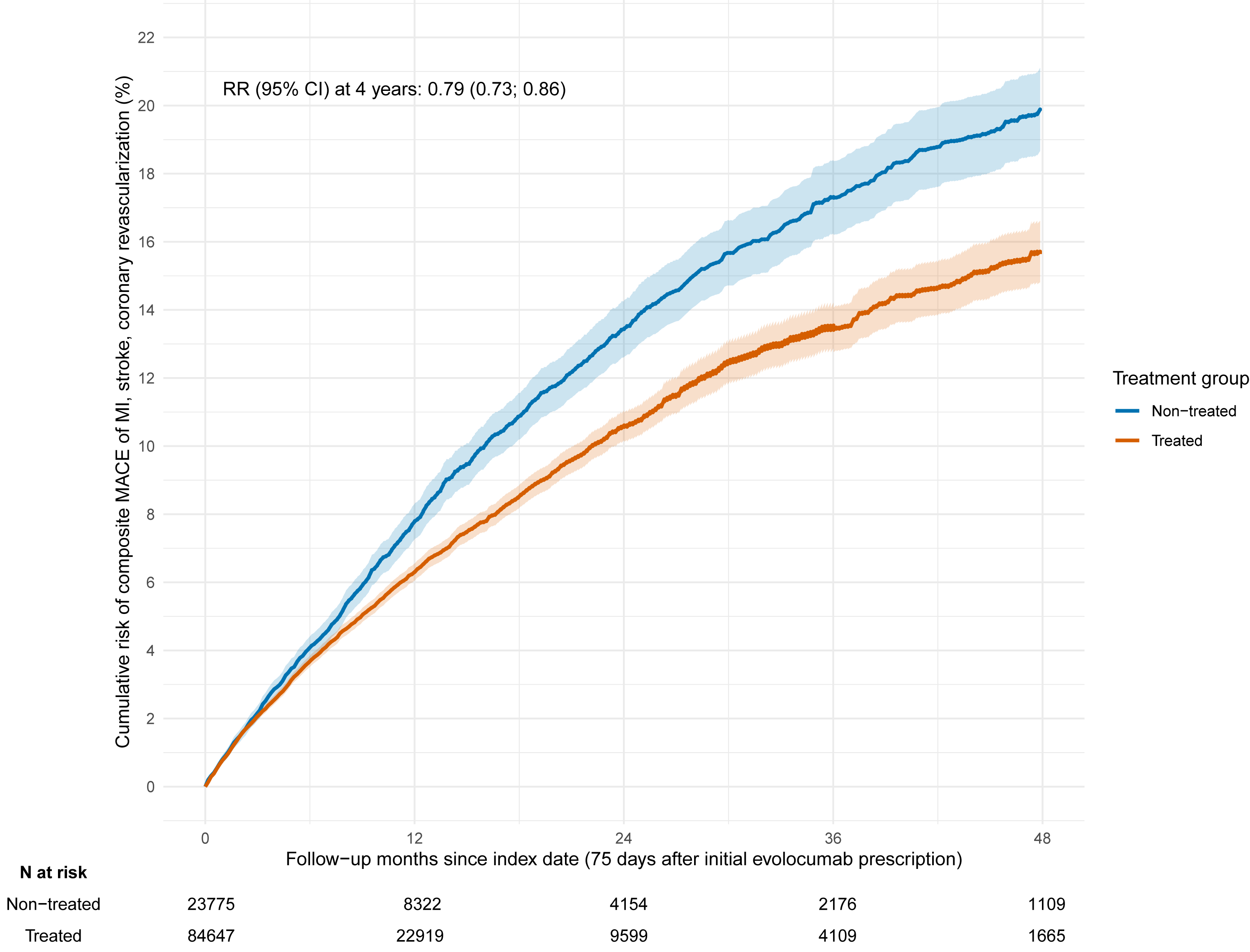
Results: The final analysis included 88,713 and 25,075 eligible patients in the treated and non-treated cohort, respectively. The weighted baseline characteristics were comparable between cohorts (Table). Patients in the treated cohort had a 21% (RR = 0.79; 95% CI; 0.73–0.86) lower risk of composite MACE at 4 years compared to those in the non-treated cohort (Figure).
Conclusions: In this RW study, evolocumab was effective in reducing MACE outcomes in patients with clinical ASCVD. The findings are consistent with the results of the FOURIER trial and extend the evidence of effectiveness of evolocumab to a larger and more diverse ASCVD cohort, with a longer follow-up period in the RW setting.
Aim: To evaluate the RW effectiveness of evolocumab in reducing MACE in patients with ASCVD.
Methods: Patients (≥ 18 years) who initiated evolocumab between 2017 and 2023 with a history of ASCVD were identified from the Komodo research database. The index date was defined as day 75 following the initial prescription of evolocumab. Patients were classified into two cohorts: the treated cohort, who initiated and remained on evolocumab, and the non-treated cohort, who initiated but discontinued the evolocumab prior to the index date. The non-treated cohort was selected to limit bias related to the initial treatment decision, leveraging the context from RCT that early discontinuation of evolocumab within the first 75 days has no known sustained clinical benefit. The primary outcome was the composite MACE of MI, stroke, and coronary revascularization. Patients were followed from the index date until the earliest of the following: occurrence of outcome, discontinuation of evolocumab (for treated cohort), reinitiation of evolocumab or other PCSK9 inhibitors (for non-treated cohort), end of database, or death. Cumulative incidence of outcome and 4-year risk ratio (RR) between treatment cohorts were calculated to evaluate the effectiveness of evolocumab, adjusting for confounders and informative censoring using inverse probability of treatment and censoring weights. Potential residual confounding was evaluated using negative controls prior to initiating the outcome analysis.
Results: The final analysis included 88,713 and 25,075 eligible patients in the treated and non-treated cohort, respectively. The weighted baseline characteristics were comparable between cohorts (Table). Patients in the treated cohort had a 21% (RR = 0.79; 95% CI; 0.73–0.86) lower risk of composite MACE at 4 years compared to those in the non-treated cohort (Figure).
Conclusions: In this RW study, evolocumab was effective in reducing MACE outcomes in patients with clinical ASCVD. The findings are consistent with the results of the FOURIER trial and extend the evidence of effectiveness of evolocumab to a larger and more diverse ASCVD cohort, with a longer follow-up period in the RW setting.
More abstracts on this topic:
A Novel RNA Interference Agent RN0191 Lowering Proprotein Convertase Subtilisin/Kexin Type 9, Low-density Lipoprotein Cholesterol and Other Lipid Biomarkers in Healthy Volunteers with Elevated LDL Cholesterol: A Randomized, Single-blind, Placebo-controlled, Phase 1 Trial
Wang Fangfang, Li Haiyan, Zeng Jie, Shi Yibin, Li Hongmei
A Community-Based Intervention to Improve Cardiovascular Health Understanding in the Dallas-Fort Worth South Asian CommunityDeo Parminder, Rohatgi Anand, Sharma Parul, Sathyamoorthy Mohanakrishnan