Final ID: MP2762
In vivo generation of CAR-M via LNP-mRNA targeting macrophages for immunotherapy in cardiac repair
Abstract Body (Do not enter title and authors here): Background
Inflammatory response is a crucial mechanism in the development and progression of heart failure following ischemic cardiac injury, partly because the delayed clearance of apoptotic cells triggers excessive inflammation. Under normal circumstances, efferocytosis by macrophages mediates the active removal of apoptotic cells. However, after cardiac injury, the loss of effector cells (macrophages) and inactivation of effector receptor function lead to impaired local efferocytosis, hindering injury repair.
Objective
This project aims to develop an in vivo strategy utilizing lipid nanoparticles (LNPs) encapsulating opsonizing antibody-conjugated mRNA for targeted delivery to monocytes/macrophages. This mRNA will induce the generation of pro-efferocytic chimeric antigen receptor macrophages (CAR-M).
Methods
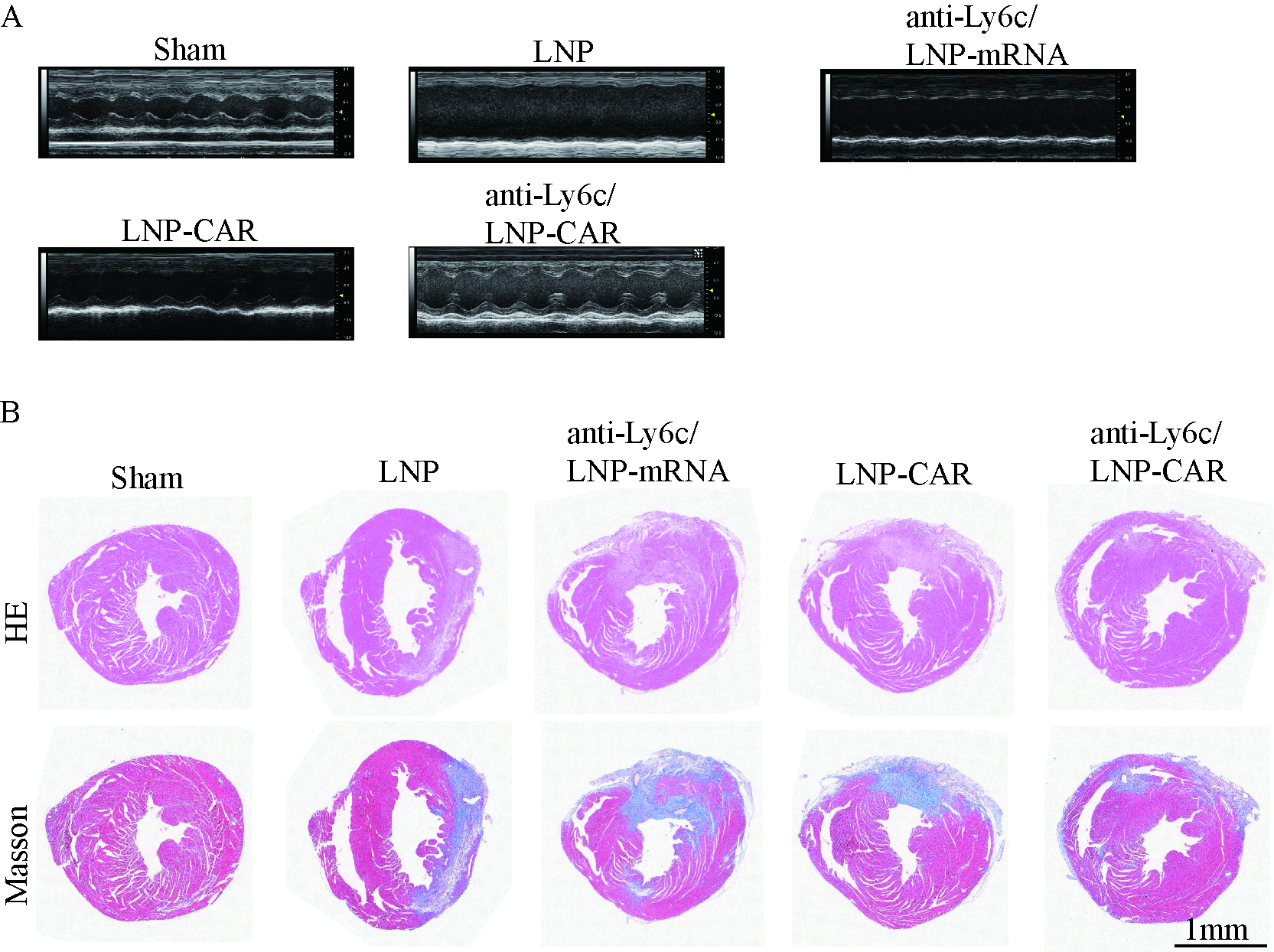
We will develop an anti-Ly6c/LNP-CAR system that delivers pro-phagocytic CAR using Ly6c antibody-conjugated lipid nanoparticles. CAR mRNA will be synthesized in vitro and encapsulated into LNPs via microfluidic mixing. CAR expression and phagocytic function in monocytes/macrophages will be assessed in vitro by qPCR, Western blot, flow cytometry, and immunofluorescence. For in vivo studies, αMHC-tdTomato transgenic mice with myocardial I/R injury will be used to evaluate targeting of Ly6C+ monocytes, their recruitment and phagocytic enhancement in injured myocardium via in vivo imaging, flow cytometry, and immunofluorescence. Cardiac repair effects will be assessed by Masson staining and
echocardiography.
Results
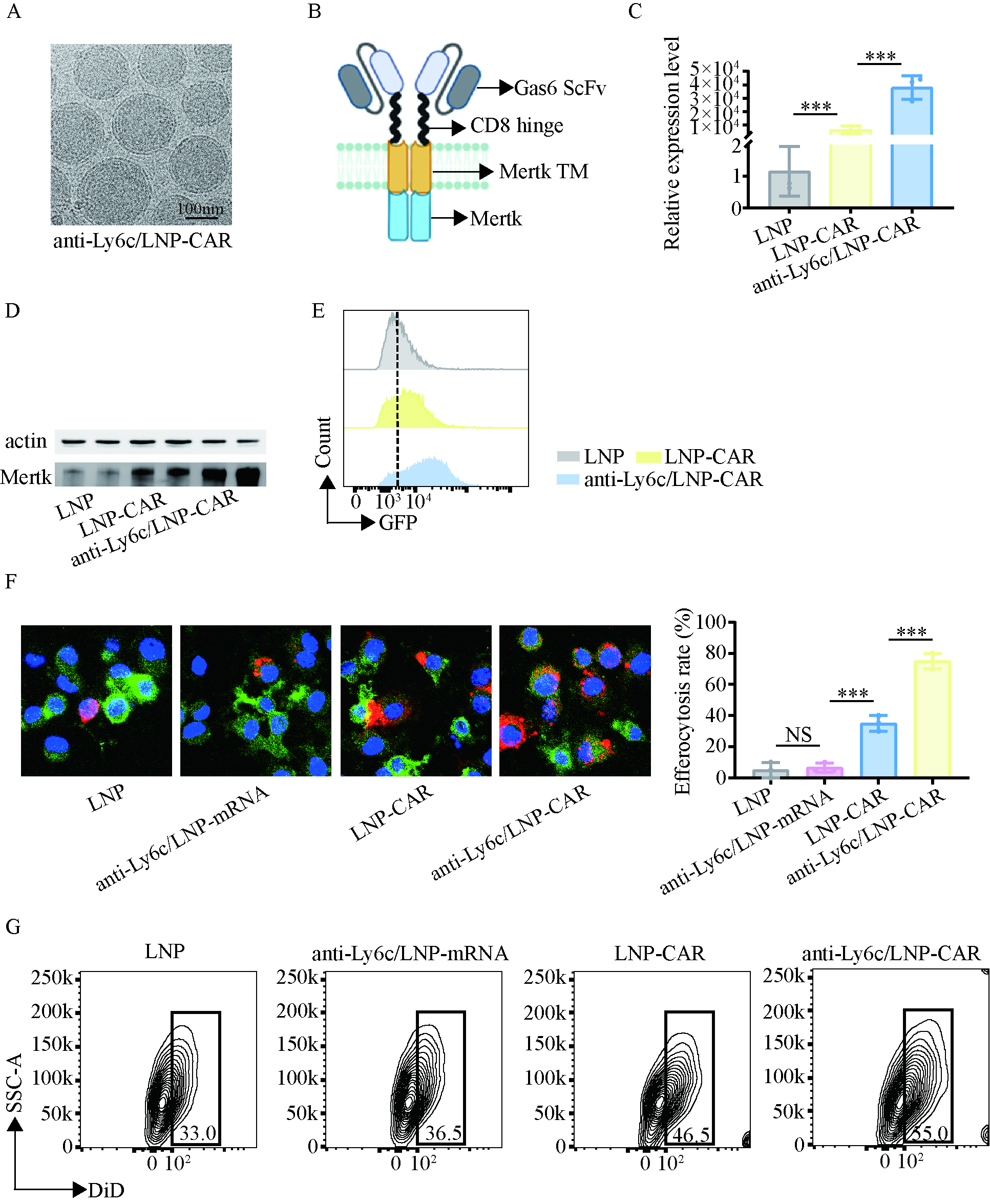
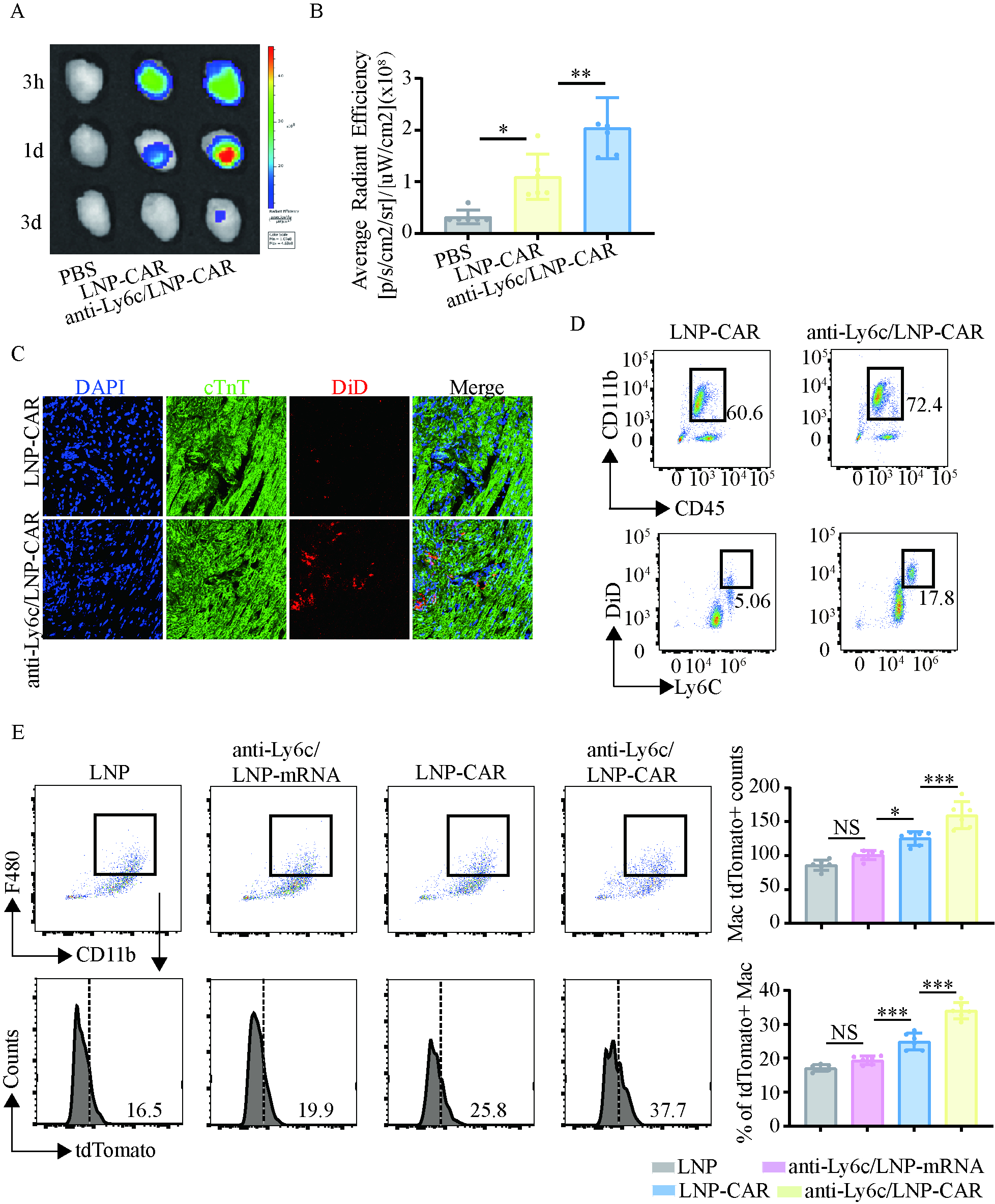
TEM confirmed well-defined anti-Ly6c/LNP-CAR nanoparticle morphology. In vitro, anti-Ly6c/LNP-CAR showed superior delivery efficiency to macrophages and enhanced phagocytic activity. In vivo, anti-Ly6c/LNP-CAR efficiently targeted monocytes and increased recruitment of macrophages to the ischemic myocardium, leading to the greatest improvement in cardiac repair.
Conclusion
This study proposes an in vivo CAR-M therapy based on an antibody-conjugated LNP platform for mRNA delivery. This in vivo CAR-M therapy organically integrates the two intrinsic causes of efferocytosis dysfunction following ischemic cardiac injury (reduced number of effector cells and cleavage-induced inactivation of effector receptors). By focusing on fundamentally reducing inflammation through promoting efferocytosis, this approach allows for more precise application of in vivo CAR-M therapy in the active regulation of local inflammation at the site of cardiac injury.
Inflammatory response is a crucial mechanism in the development and progression of heart failure following ischemic cardiac injury, partly because the delayed clearance of apoptotic cells triggers excessive inflammation. Under normal circumstances, efferocytosis by macrophages mediates the active removal of apoptotic cells. However, after cardiac injury, the loss of effector cells (macrophages) and inactivation of effector receptor function lead to impaired local efferocytosis, hindering injury repair.
Objective
This project aims to develop an in vivo strategy utilizing lipid nanoparticles (LNPs) encapsulating opsonizing antibody-conjugated mRNA for targeted delivery to monocytes/macrophages. This mRNA will induce the generation of pro-efferocytic chimeric antigen receptor macrophages (CAR-M).
Methods
We will develop an anti-Ly6c/LNP-CAR system that delivers pro-phagocytic CAR using Ly6c antibody-conjugated lipid nanoparticles. CAR mRNA will be synthesized in vitro and encapsulated into LNPs via microfluidic mixing. CAR expression and phagocytic function in monocytes/macrophages will be assessed in vitro by qPCR, Western blot, flow cytometry, and immunofluorescence. For in vivo studies, αMHC-tdTomato transgenic mice with myocardial I/R injury will be used to evaluate targeting of Ly6C+ monocytes, their recruitment and phagocytic enhancement in injured myocardium via in vivo imaging, flow cytometry, and immunofluorescence. Cardiac repair effects will be assessed by Masson staining and
echocardiography.
Results
TEM confirmed well-defined anti-Ly6c/LNP-CAR nanoparticle morphology. In vitro, anti-Ly6c/LNP-CAR showed superior delivery efficiency to macrophages and enhanced phagocytic activity. In vivo, anti-Ly6c/LNP-CAR efficiently targeted monocytes and increased recruitment of macrophages to the ischemic myocardium, leading to the greatest improvement in cardiac repair.
Conclusion
This study proposes an in vivo CAR-M therapy based on an antibody-conjugated LNP platform for mRNA delivery. This in vivo CAR-M therapy organically integrates the two intrinsic causes of efferocytosis dysfunction following ischemic cardiac injury (reduced number of effector cells and cleavage-induced inactivation of effector receptors). By focusing on fundamentally reducing inflammation through promoting efferocytosis, this approach allows for more precise application of in vivo CAR-M therapy in the active regulation of local inflammation at the site of cardiac injury.
More abstracts on this topic:
A Novel Multivariate Scoring System for Diagnosing Post-Myocardial Infarction Pericarditis Following Percutaneous Coronary Intervention
Bolaji Olayiwola, Omoru Okiemute, Upreti Prakash, Echari Blanche, Shoar Saeed, Basit Jawad, Alraies M Chadi
A novel reproducible low-cost model of acute myocardial infarction in swineLi Yichen, Zheng Zilong, Tang Weijie, Chen Wangping, Yang Jinfu, Fan Chengming