Final ID: MP1138
Finerenone Attenuates Cardiac Hypertrophy in a Mouse Model of HFpEF by Modulating Glucocorticoid and Mineralocorticoid Receptor Signaling
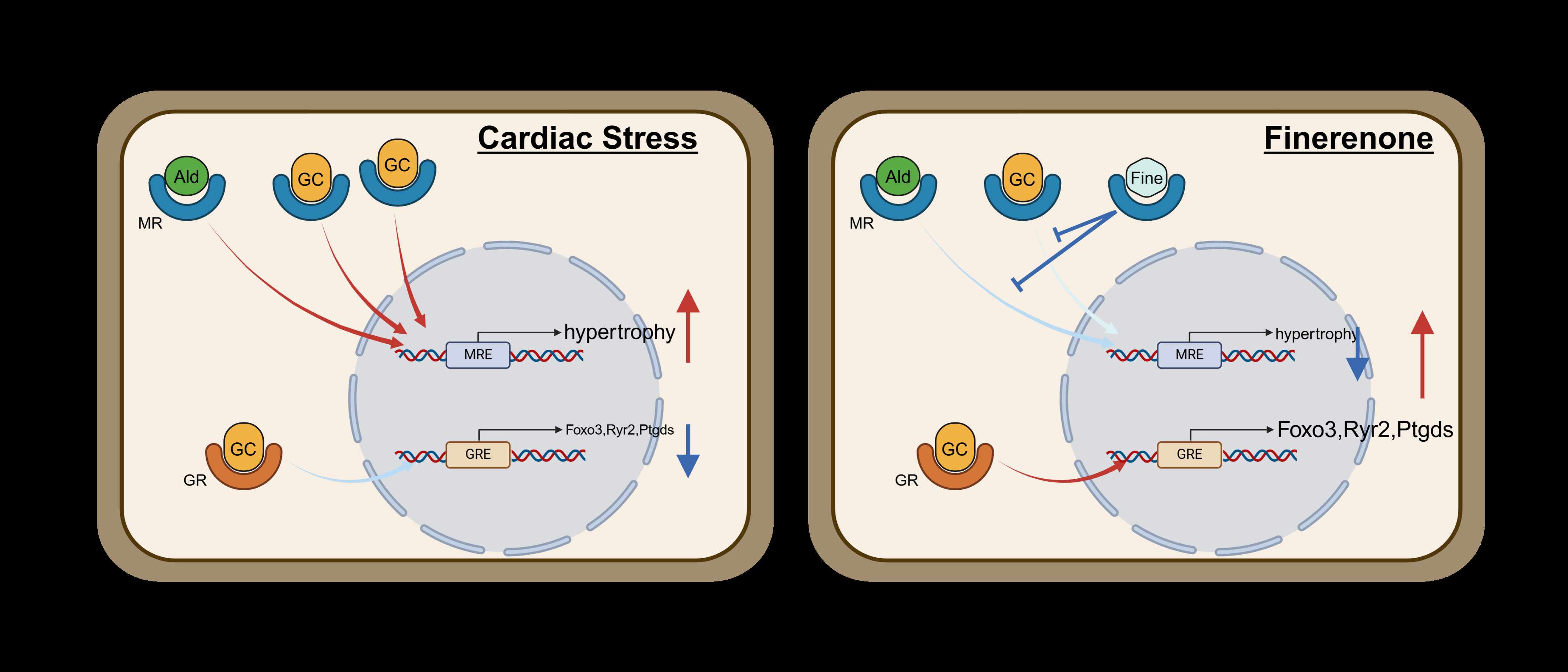
Abstract Body (Do not enter title and authors here): Background: The FINEARTS-HF trial demonstrated that finerenone significantly reduced cardiovascular death and total heart failure events in patients with mildly reduced or preserved ejection fraction compared to placebo. However, the pharmacological mechanisms by which this highly selective, non-steroidal mineralocorticoid receptor antagonist (MRA) exerts cardioprotective effects in heart failure with preserved ejection fraction (HFpEF) remain insufficiently defined. Recent studies have indicated that mineralocorticoid receptor (MR) and glucocorticoid receptor (GR) not only interact but also mutually regulate their signaling across multiple organs, including the myocardium.
Methods: A murine HFpEF model was established by inducing obesity, diabetes, and metabolic dysfunction-associated fatty liver disease (MAFLD) in conjunction with hypertension. Wild-type C57BL/6J mice were fed a high-fat diet and administered NG-nitro-L-arginine methyl ester (L-NAME) for 10 weeks. During the pathological hypertrophy phase, mice were treated with either finerenone or placebo for two weeks via oral gavage. Cardiac function and arterial pressure were evaluated using transthoracic echocardiography and continuous invasive blood pressure monitoring. Myocardial tissue underwent histological evaluation, immunoblotting, and transcriptomic analysis from cardiomyocyte-enriched nuclear fractions to elucidate finerenone-specific cardiac effects.
Results: Combined metabolic and hypertensive stress induced obesity, glucose intolerance, elevated blood pressure, and MAFLD, alongside increased MR nuclear localization in cardiomyocytes. Despite no significant group difference in 24-hour arterial pressure, finerenone-treated mice exhibited significantly reduced cardiomyocyte cross-sectional area (placebo: 235.9 ± 41.7 μm^2; finerenone: 213.7 ± 60.3 μm^2; p = 0.0189) and preserved capillary density. RNA sequencing revealed that finerenone suppressed expression of hypertrophic gene signatures while maintaining GR target gene expression associated with cardiac resilience. Protein analysis confirmed that GR nuclear translocation was preserved in the finerenone group.
Conclusion: Finerenone mitigates cardiomyocyte hypertrophy in a metabolic HFpEF model, independent of blood pressure reduction. This effect may be attributable to rebalancing MR and GR signaling in the myocardium, offering novel insight into finerenone’s mode of action beyond mineralocorticoid blockade.
Methods: A murine HFpEF model was established by inducing obesity, diabetes, and metabolic dysfunction-associated fatty liver disease (MAFLD) in conjunction with hypertension. Wild-type C57BL/6J mice were fed a high-fat diet and administered NG-nitro-L-arginine methyl ester (L-NAME) for 10 weeks. During the pathological hypertrophy phase, mice were treated with either finerenone or placebo for two weeks via oral gavage. Cardiac function and arterial pressure were evaluated using transthoracic echocardiography and continuous invasive blood pressure monitoring. Myocardial tissue underwent histological evaluation, immunoblotting, and transcriptomic analysis from cardiomyocyte-enriched nuclear fractions to elucidate finerenone-specific cardiac effects.
Results: Combined metabolic and hypertensive stress induced obesity, glucose intolerance, elevated blood pressure, and MAFLD, alongside increased MR nuclear localization in cardiomyocytes. Despite no significant group difference in 24-hour arterial pressure, finerenone-treated mice exhibited significantly reduced cardiomyocyte cross-sectional area (placebo: 235.9 ± 41.7 μm^2; finerenone: 213.7 ± 60.3 μm^2; p = 0.0189) and preserved capillary density. RNA sequencing revealed that finerenone suppressed expression of hypertrophic gene signatures while maintaining GR target gene expression associated with cardiac resilience. Protein analysis confirmed that GR nuclear translocation was preserved in the finerenone group.
Conclusion: Finerenone mitigates cardiomyocyte hypertrophy in a metabolic HFpEF model, independent of blood pressure reduction. This effect may be attributable to rebalancing MR and GR signaling in the myocardium, offering novel insight into finerenone’s mode of action beyond mineralocorticoid blockade.
More abstracts on this topic:
A First-in-Class Humanized Antibody Fragment Targeting Platelet Glycoprotein Ibα: A Comprehensive Preclinical Study of CA1001 for the Treatment of Acute Ischemic Stroke
Xu Xiaohong, Preeti Preeti, Yu Ruoying, Shaykhalishahi Hamed, Zhang Cheng, Shen Chuanbin, Li Bei, Tang Naping, Chang Yan, Xiang Qian, Cui Yimin, Lei Xi, Ni Heyu, Zhu Guangheng, Liu Zhenze, Hu Xudong, Slavkovic Sladjana, Neves Miguel, Ma Wenjing, Xie Huifang
4-Hydroxy-2-Nonenal Alters Alternative Polyadenylation to Regulate mRNA Isoform Diversity in the Transition from Human Cardiac Fibroblasts to MyofibroblastsNatarajan Kartiga, Neupane Rahul, Yalamanchili Hari Krishna, Palaniyandi Suresh, Wagner Eric, Guha Ashrith, Amirthalingam Thandavarayan Rajarajan